Korean J Radiol.
2018 Oct;19(5):897-904. 10.3348/kjr.2018.19.5.897.
Identification of Preoperative Magnetic Resonance Imaging Features Associated with Positive Resection Margins in Breast Cancer: A Retrospective Study
- Affiliations
-
- 1Department of Radiology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul 06273, Korea. EJSONRD@yuhs.ac
- 2Department of Diagnostic Radiology, CHA Bundang Medical Center, CHA University, Seongnam 13496, Korea.
- KMID: 2418553
- DOI: http://doi.org/10.3348/kjr.2018.19.5.897
Abstract
OBJECTIVE
To determine which preoperative breast magnetic resonance imaging (MRI) findings and clinicopathologic features are associated with positive resection margins at the time of breast-conserving surgery (BCS) in patients with breast cancer.
MATERIALS AND METHODS
We reviewed preoperative breast MRI and clinicopathologic features of 120 patients (mean age, 53.3 years; age range, 27-79 years) with breast cancer who had undergone BCS in 2015. Tumor size on MRI, multifocality, patterns of enhancing lesions (mass without non-mass enhancement [NME] vs. NME with or without mass), mass characteristics (shape, margin, internal enhancement characteristics), NME (distribution, internal enhancement patterns), and breast parenchymal enhancement (BPE; weak, strong) were analyzed. We also evaluated age, tumor size, histology, lymphovascular invasion, T stage, N stage, and hormonal receptors. Univariate and multivariate logistic regression analyses were used to determine the correlation between clinicopathological features, MRI findings, and positive resection margins.
RESULTS
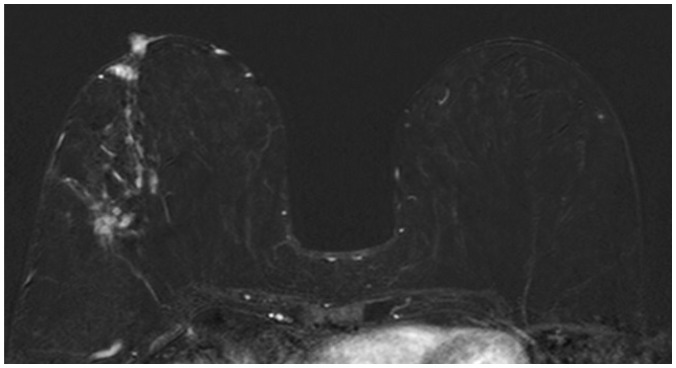
In univariate analysis, tumor size on MRI, multifocality, NME with or without mass, and segmental distribution of NME were correlated with positive resection margins. Among the clinicopathological factors, tumor size of the invasive breast cancer and in situ components were significantly correlated with a positive resection margin. Multivariate analysis revealed that NME with or without mass was an independent predictor of positive resection margins (odds ratio [OR] = 7.00; p < 0.001). Strong BPE was a weak predictor of positive resection margins (OR = 2.59; p = 0.076).
CONCLUSION
Non-mass enhancement with or without mass is significantly associated with a positive resection margin in patients with breast cancer. In patients with NME, segmental distribution was significantly correlated with positive resection margins.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Re: Identification of Preoperative Magnetic Resonance Imaging Features Associated with Positive Resection Margins in Breast Cancer?
Dongzhi Cen, Wanyan Hu, Xuelin Wang, Xiaohuan Wu
Korean J Radiol. 2019;20(6):999-1000. doi: 10.3348/kjr.2019.0044.
Reference
-
1. Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002; 347:1233–1241. PMID: 12393820.
Article2. Veronesi U, Cascinelli N, Mariani L, Greco M, Saccozzi R, Luini A, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002; 347:1227–1232. PMID: 12393819.
Article3. Singletary SE. Surgical margins in patients with early-stage breast cancer treated with breast conservation therapy. Am J Surg. 2002; 184:383–393. PMID: 12433599.
Article4. Houssami N, Macaskill P, Marinovich ML, Dixon JM, Irwig L, Brennan ME, et al. Meta-analysis of the impact of surgical margins on local recurrence in women with early-stage invasive breast cancer treated with breast-conserving therapy. Eur J Cancer. 2010; 46:3219–3232. PMID: 20817513.
Article5. Park AY, Seo BK. Real-time MRI navigated ultrasound for preoperative tumor evaluation in breast cancer patients: technique and clinical implementation. Korean J Radiol. 2016; 17:695–705. PMID: 27587958.
Article6. Azu M, Abrahamse P, Katz SJ, Jagsi R, Morrow M. What is an adequate margin for breast-conserving surgery? Surgeon attitudes and correlates. Ann Surg Oncol. 2010; 17:558–563. PMID: 19847566.
Article7. Bani MR, Lux MP, Heusinger K, Wenkel E, Magener A, Schulz-Wendtland R, et al. Factors correlating with reexcision after breast-conserving therapy. Eur J Surg Oncol. 2009; 35:32–37. PMID: 18539425.
Article8. Lovrics PJ, Cornacchi SD, Farrokhyar F, Garnett A, Chen V, Franic S, et al. The relationship between surgical factors and margin status after breast-conservation surgery for early stage breast cancer. Am J Surg. 2009; 197:740–746. PMID: 18789424.
Article9. Park S, Park HS, Kim SI, Koo JS, Park BW, Lee KS. The impact of a focally positive resection margin on the local control in patients treated with breast-conserving therapy. Jpn J Clin Oncol. 2011; 41:600–608. PMID: 21355001.
Article10. Melstrom LG, Melstrom KA, Wang EC, Pilewskie M, Winchester DJ. Ductal carcinoma in situ: size and resection volume predict margin status. Am J Clin Oncol. 2010; 33:438–442. PMID: 20023569.11. Seo M, Cho N, Bae MS, Koo HR, Kim WH, Lee SH, et al. Features of undiagnosed breast cancers at screening breast MR imaging and potential utility of computer-aided evaluation. Korean J Radiol. 2016; 17:59–68. PMID: 26798217.
Article12. Chen SQ, Huang M, Shen YY, Liu CL, Xu CX. Abbreviated MRI protocols for detecting breast cancer in women with dense breasts. Korean J Radiol. 2017; 18:470–475. PMID: 28458599.
Article13. Chung A, Saouaf R, Scharre K, Phillips E. The impact of MRI on the treatment of DCIS. Am Surg. 2005; 71:705–710. PMID: 16468502.
Article14. Del Frate C, Borghese L, Cedolini C, Bestagno A, Puglisi F, Isola M, et al. Role of pre-surgical breast MRI in the management of invasive breast carcinoma. Breast. 2007; 16:469–481. PMID: 17433681.
Article15. Schnall M. MR imaging evaluation of cancer extent: is there clinical relevance? Magn Reson Imaging Clin N Am. 2006; 14:379–381. viiPMID: 17098178.
Article16. Wolff AC, Hammond ME, Schwartz JN, Hagerty KL, Allred DC, Cote RJ, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007; 25:118–145. PMID: 17159189.
Article17. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, editors. AJCC cancer staging manual. 7th ed. New York, NY: Springer;2010.18. D'Orsi CJ, Sickles EA, Mendelson EB, Morris EA. ACR BI-RADS atlas, breast imaging reporting and data system. Reston, VA: American College of Radiology;2013.19. Camp ER, McAuliffe PF, Gilroy JS, Morris CG, Lind DS, Mendenhall NP, et al. Minimizing local recurrence after breast conserving therapy using intraoperative shaved margins to determine pathologic tumor clearance. J Am Coll Surg. 2005; 201:855–861. PMID: 16310688.
Article20. Turnbull L, Brown S, Harvey I, Olivier C, Drew P, Napp V, et al. Comparative effectiveness of MRI in breast cancer (COMICE) trial: a randomised controlled trial. Lancet. 2010; 375:563–571. PMID: 20159292.
Article21. Houssami N, Turner R, Morrow M. Preoperative magnetic resonance imaging in breast cancer: meta-analysis of surgical outcomes. Ann Surg. 2013; 257:249–255. PMID: 23187751.22. Pengel KE, Loo CE, Teertstra HJ, Muller SH, Wesseling J, Peterse JL, et al. The impact of preoperative MRI on breast-conserving surgery of invasive cancer: a comparative cohort study. Breast Cancer Res Treat. 2009; 116:161–169. PMID: 18807269.
Article23. Mann RM, Loo CE, Wobbes T, Bult P, Barentsz JO, Gilhuijs KG, et al. The impact of preoperative breast MRI on the re-excision rate in invasive lobular carcinoma of the breast. Breast Cancer Res Treat. 2010; 119:415–422. PMID: 19885731.
Article24. Kim OH, Kim SJ, Lee JS. Enhancing patterns of breast cancer on preoperative dynamic contrast-enhanced magnetic resonance imaging and resection margin in breast conserving therapy. Breast Dis. 2016; 36:27–35. PMID: 27177341.
Article25. Jang M, Kim SM, Yun BL, Kim SW, Kang EY, Park SY, et al. Magnetic resonance imaging factors predicting re-excision in breast cancer patients having undergone conserving therapy. J Korean Soc Magn Reson Med. 2014; 18:133–143.
Article26. Sakamoto N, Tozaki M, Higa K, Tsunoda Y, Ogawa T, Abe S, et al. Categorization of non-mass-like breast lesions detected by MRI. Breast Cancer. 2008; 15:241–246. PMID: 18224381.
Article27. Tozaki M, Fukuda K. High-spatial-resolution MRI of non-masslike breast lesions: interpretation model based on BI-RADS MRI descriptors. AJR Am J Roentgenol. 2006; 187:330–337. PMID: 16861534.
Article28. Vag T, Baltzer PA, Dietzel M, Benndorf M, Gajda M, Camara O, et al. Kinetic characteristics of ductal carcinoma in situ (DCIS) in dynamic breast MRI using computer-assisted analysis. Acta Radiol. 2010; 51:955–961. PMID: 20942728.
Article29. Dillon MF, Hill AD, Quinn CM, McDermott EW, O'Higgins N. A pathologic assessment of adequate margin status in breast-conserving therapy. Ann Surg Oncol. 2006; 13:333–339. PMID: 16474911.
Article30. Miller AR, Brandao G, Prihoda TJ, Hill C, Cruz AB Jr, Yeh IT. Positive margins following surgical resection of breast carcinoma: analysis of pathologic correlates. J Surg Oncol. 2004; 86:134–140. PMID: 15170651.
Article31. DiPiro PJ, Krajewski KM, Giardino AA, Braschi-Amirfarzan M, Ramaiya NH. Radiology consultation in the era of precision oncology: a review of consultation models and services in the tertiary setting. Korean J Radiol. 2017; 18:18–27. PMID: 28096715.
Article32. Seo M, Ryu JK, Jahng GH, Sohn YM, Rhee SJ, Oh JH, et al. Estimation of T2* relaxation time of breast cancer: correlation with clinical, imaging and pathological features. Korean J Radiol. 2017; 18:238–248. PMID: 28096732.33. Park SY, Kang DK, Kim TH. Does background parenchymal enhancement on MRI affect the rate of positive resection margin in breast cancer patients? Br J Radiol. 2015; 88:20140638. PMID: 25429418.
Article34. Millet I, Pages E, Hoa D, Merigeaud S, Curros Doyon F, Prat X, et al. Pearls and pitfalls in breast MRI. Br J Radiol. 2012; 85:197–207. PMID: 22128131.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Re: Identification of Preoperative Magnetic Resonance Imaging Features Associated with Positive Resection Margins in Breast Cancer?
- Preoperative Magnetic Resonance Imaging FeaturesAssociated with Positive Resection Margins in Patientswith Invasive Lobular Carcinoma
- Clinical Significance of Non-Mass-Like Enhancement of Preoperative Magnetic Resonance Imaging in Breast Cancer Considering Breast-Conserving Surgery
- A Rare Case of Male Primary Breast Lymphoma
- The Role of Preoperative Breast MRI in Patients With Early-Stage Breast Cancer