Korean J Orthod.
2018 May;48(3):163-171. 10.4041/kjod.2018.48.3.163.
Antibacterial and remineralization effects of orthodontic bonding agents containing bioactive glass
- Affiliations
-
- 1Department of Orthodontics, Dental Research Institute, Pusan National University Dental Hospital, Yangsan, Korea. kimyongil@pusan.ac.kr
- 2R&D Center, Upex·med Co., Ltd., Anyang, Korea.
- 3School of Materials Science and Engineering, Pusan National University, Busan, Korea.
- 4Department of Oral Microbiology, School of Dentistry, Pusan National University, Yangsan, Korea.
- 5Institute of Translational Dental Sciences, School of Dentistry, Pusan National University, Yangsan, Korea.
- 6Department of Dental Materials, Pusan National University, Yangsan, Korea. y0k0916@pusan.ac.kr
- KMID: 2418327
- DOI: http://doi.org/10.4041/kjod.2018.48.3.163
Abstract
OBJECTIVE
The aim of this study was to evaluate the mechanical and biological properties of orthodontic bonding agents containing silver- or zinc-doped bioactive glass (BAG) and determine the antibacterial and remineralization effects of these agents.
METHODS
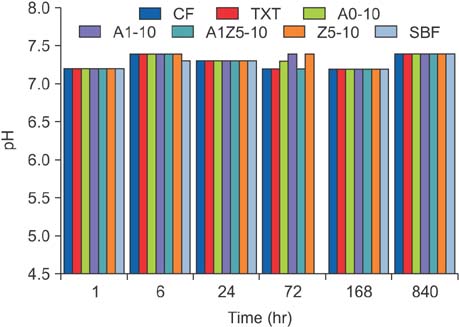
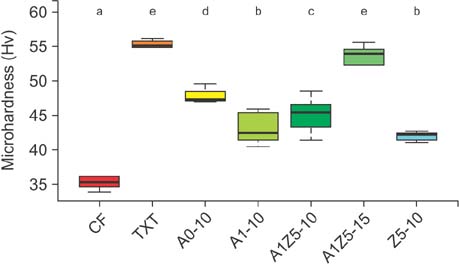
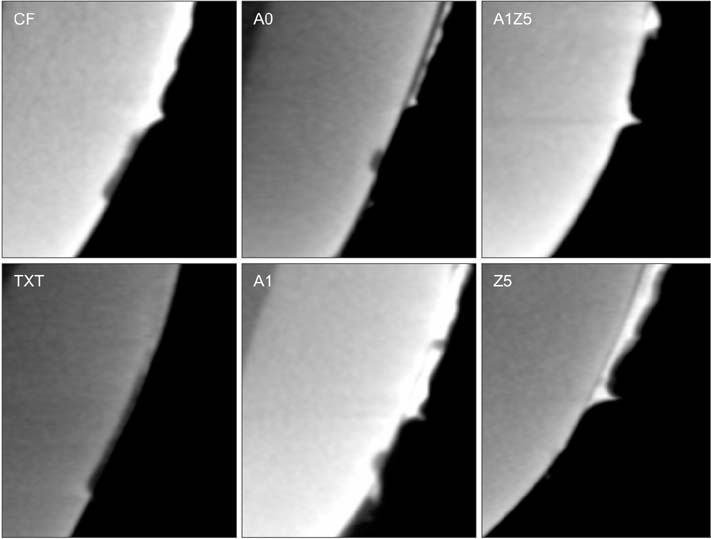
BAG was synthesized using the alkali-mediated solgel method. Orthodontic bonding agents containing BAG were prepared by mixing BAG with flowable resin. Transbondâ„¢ XT (TXT) and Charmfilâ„¢ Flow (CF) were used as controls. Ion release, cytotoxicity, antibacterial properties, the shear bond strength, and the adhesive remnant index were evaluated. To assess the remineralization properties of BAG, micro-computed tomography was performed after pH cycling.
RESULTS
The BAG-containing bonding agents showed no noticeable cytotoxicity and suppressed bacterial growth. When these bonding agents were used, demineralization after pH cycling began approximately 200 to 300 µm away from the bracket. On the other hand, when CF and TXT were used, all surfaces that were not covered by the adhesive were demineralized after pH cycling.
CONCLUSIONS
Our findings suggest that orthodontic bonding agents containing silver- or zinc-doped BAG have stronger antibacterial and remineralization effects compared with conventional orthodontic adhesives; thus, they are suitable for use in orthodontic practice.
Keyword
MeSH Terms
Figure
Reference
-
1. Bishara SE, Ostby AW. White spot lesions: formation, prevention, and treatment. Semin Orthod. 2008; 14:174–182.
Article2. Hashimoto M, Iijima M, Nagano F, Ohno H, Endo K. Effect of monomer composition on crystal growth by resin containing bioglass. J Biomed Mater Res B Appl Biomater. 2010; 94:127–133.
Article3. Manfred L, Covell DA, Crowe JJ, Tufekci E, Mitchell JC. A novel biomimetic orthodontic bonding agent helps prevent white spot lesions adjacent to brackets. Angle Orthod. 2013; 83:97–103.
Article4. Chatzistavrou X, Velamakanni S, DiRenzo K, Lefkelidou A, Fenno JC, Kasuga T, et al. Designing dental composites with bioactive and bactericidal properties. Mater Sci Eng C Mater Biol Appl. 2015; 52:267–272.
Article5. Hench LL. The story of bioglass. J Mater Sci Mater Med. 2006; 17:967–978.
Article6. Jones JR. Review of bioactive glass: from Hench to hybrids. Acta Biomater. 2013; 9:4457–4486.
Article7. Abbasi Z, Bahrololoom ME, Shariat MH, Bagheri R. Bioactive glasses in dentistry: a review. J Dent Biomater. 2015; 2:1–9.8. Bellantone M, Williams HD, Hench LL. Broad-spectrum bactericidal activity of Ag(2)O-doped bioactive glass. Antimicrob Agents Chemother. 2002; 46:1940–1945.
Article9. Wang YY, Chatzistavrou X, Faulk D, Badylak S, Zheng L, Papagerakis S, et al. Biological and bactericidal properties of Ag-doped bioactive glass in a natural extracellular matrix hydrogel with potential application in dentistry. Eur Cell Mater. 2015; 29:342–355.
Article10. Balasubramanian P, Strobel LA, Kneser U, Boccaccini AR. Zinc-containing bioactive glasses for bone regeneration, dental and orthopedic applications. Biomed Glasses. 2015; 1:51–69.
Article11. Goh YF, Alshemary AZ, Akram M, Kadir MRA, Hussian R. Bioactive glass: an in-vitro comparative study of doping with nanoscale copper and silver particles. Int J Appl Glass Sci. 2014; 5:255–266.
Article12. Xia W, Chang J. Preparation and characterization of nano-bioactive-glasses (NBG) by a quick alkalimediated sol-gel method. Mater Lett. 2007; 61:3251–3253.
Article13. Stookey GK, Featherstone JD, Rapozo-Hilo M, Schemehorn BR, Williams RA, Baker RA, et al. The featherstone laboratory pH cycling model: a prospective, multi-site validation exercise. Am J Dent. 2011; 24:322–328.14. Paschos E, Kleinschrodt T, Clementino-Luedemann T, Huth KC, Hickel R, Kunzelmann KH, et al. Effect of different bonding agents on prevention of enamel demineralization around orthodontic brackets. Am J Orthod Dentofacial Orthop. 2009; 135:603–612.
Article15. Venugopal A, Muthuchamy N, Tejani H, Gopalan AI, Lee KP, Lee HJ, et al. Incorporation of silver nanoparticles on the surface of orthodontic microimplants to achieve antimicrobial properties. Korean J Orthod. 2017; 47:3–10.
Article16. Percival SL, Bowler PG, Russell D. Bacterial resistance to silver in wound care. J Hosp Infect. 2005; 60:1–7.
Article17. Stoor P, Söderling E, Salonen JI. Antibacterial effects of a bioactive glass paste on oral microorganisms. Acta Odontol Scand. 1998; 5:161–165.
Article18. Boyd D, Li H, Tanner DA, Towler MR, Wall JG. The antibacterial effects of zinc ion migration from zinc-based glass polyalkenoate cements. J Mater Sci Mater Med. 2006; 17:489–494.
Article19. Osorio R, Cabello I, Toledano M. Bioactivity of zincdoped dental adhesives. J Dent. 2014; 42:403–412.
Article20. Brown ML, Davis HB, Tufekci E, Crowe JJ, Covell DA, Mitchell JC. Ion release from a novel orthodontic resin bonding agent for the reduction and/or prevention of white spot lesions. An in vitro study. Angle Orthod. 2011; 81:1014–1020.
Article21. Salehi S, Gwinner F, Mitchell JC, Pfeifer C, Ferracane JL. Cytotoxicity of resin composites containing bioactive glass fillers. Dent Mater. 2015; 31:195–203.
Article22. Li F, Weir MD, Chen J, Xu HH. Comparison of quaternary ammonium-containing with nano-silvercontaining adhesive in antibacterial properties and cytotoxicity. Dent Mater. 2013; 29:450–461.
Article23. Kang YG, Kim JY, Kim J, Won PJ, Nam JH. Release of bisphenol A from resin composite used to bond orthodontic lingual retainers. Am J Orthod Dentofacial Orthop. 2011; 140:779–789.
Article24. Reynolds IR, von Fraunhofer JA. Direct bonding of orthodontic brackets--a comparative study of adhesives. Br J Orthod. 1976; 3:143–146.
Article25. Park SB, Son WS, Ko CC, García-Godoy F, Park MG, Kim HI, et al. Influence of flowable resins on the shear bond strength of orthodontic brackets. Dent Mater J. 2009; 28:730–734.
Article26. Ryou DB, Park HS, Kim KH, Kwon TY. Use of flowable composites for orthodontic bracket bonding. Angle Orthod. 2008; 78:1105–1109.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effects of crystal growth on shear bond strength of orthodontic bracket adhesives to enamel surface
- The shear bond strength of two adhesives bonded to composite resin and glass ionomer cement restorations
- Galla chinensis extracts and calcium induce remineralization and antibacterial effects of enamel in a Streptococcus mutans biofilm model
- Histological Observations on Bone Healing with Bioactive Glass in Horizontal Ridge Augmentation: A Report of Four Cases
- Tensile bond strength of glass ionomer cements