Korean J Ophthalmol.
2018 Aug;32(4):328-338. 10.3341/kjo.2017.0079.
Effects of Ranibizumab, Bevacizumab, and Aflibercept on Senescent Retinal Pigment Epithelial Cells
- Affiliations
-
- 1Department of Medical Science, Konyang University College of Medicine, Daejeon, Korea. lyujm@konyang.ac.kr
- 2Department of Ophthalmology and Visual Science, The Catholic University of Korea College of Medicine, Seoul, Korea. john0730@catholic.ac.kr
- 3Myunggok Eye Research Institute, Konyang University College of Medicine, Daejeon, Korea.
- 4Clinical Research Institute, Daejeon St. Mary's Hospital, The Catholic University of Korea College of Medicine, Daejeon, Korea.
- KMID: 2418154
- DOI: http://doi.org/10.3341/kjo.2017.0079
Abstract
- PURPOSE
Anti-vascular endothelial growth factor (VEGF) agents have been used for the last 10 years, but their safety profile, including cytotoxicity against various ocular cells such as retinal pigment epithelial (RPE) cells, remains a serious concern. Safety studies of VEGF agents conducted to date have primarily relied on healthy RPE cells. In this study, we assessed the safety of three anti-VEGF agents, namely, ranibizumab, bevacizumab, and aflibercept, on senescent RPE cells.
METHODS
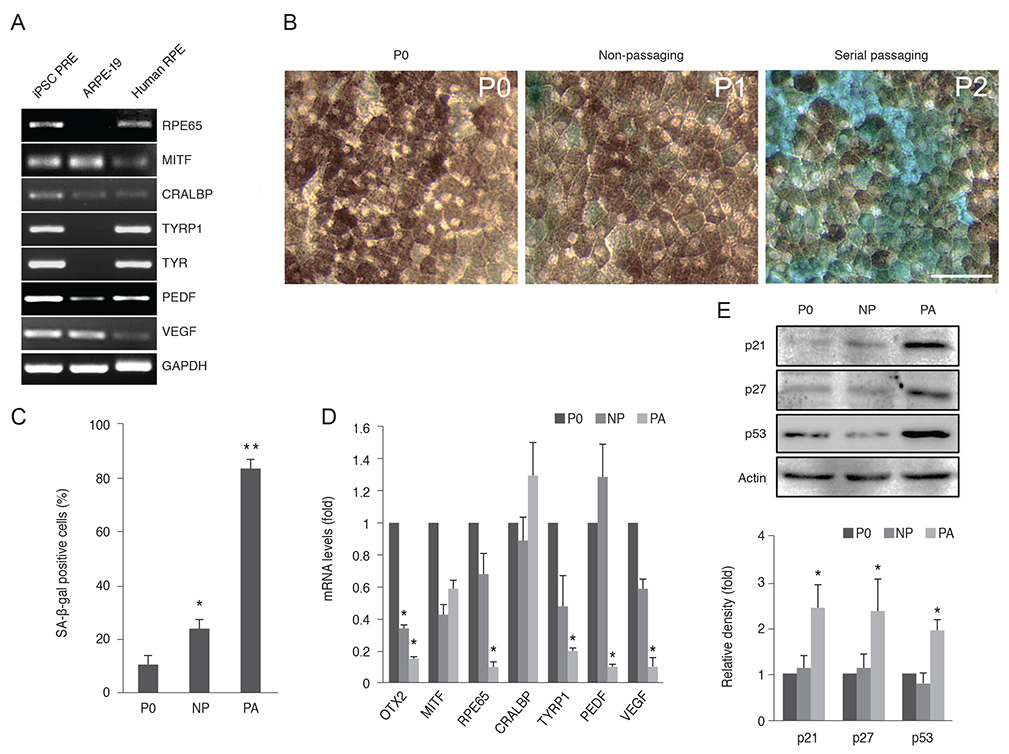
Senescent human induced pluripotent stem cell-derived RPE cells were generated by continuous replication and confirmed with senescence biomarkers. The viability, proliferation, protein expression, and phagocytosis of the senescent RPE cells were characterized 3 days after anti-VEGF treatment with clinical doses of ranibizumab, bevacizumab, or aflibercept.
RESULTS
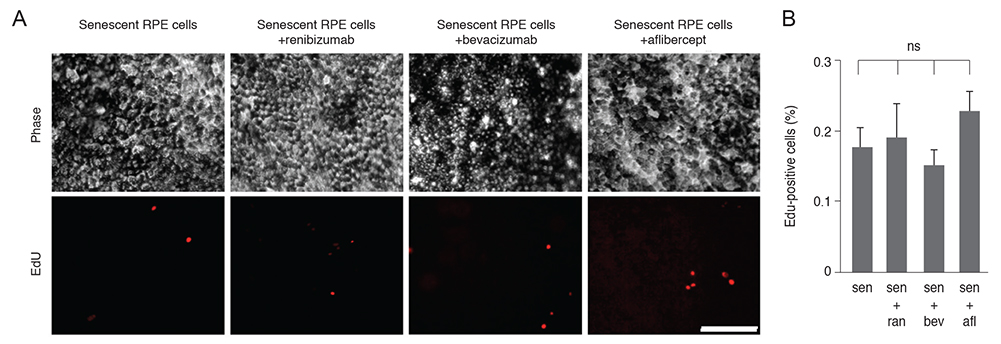
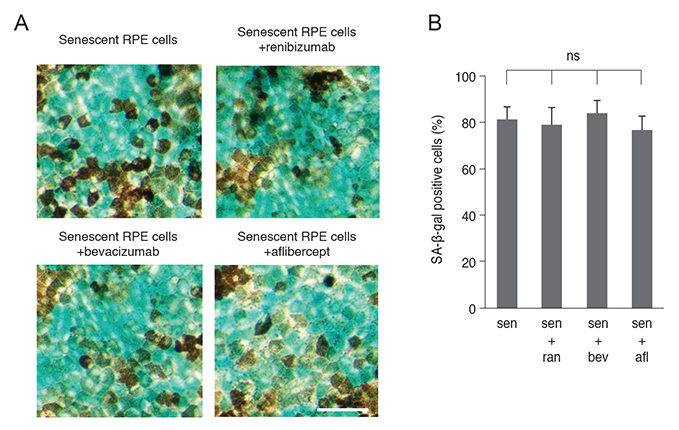
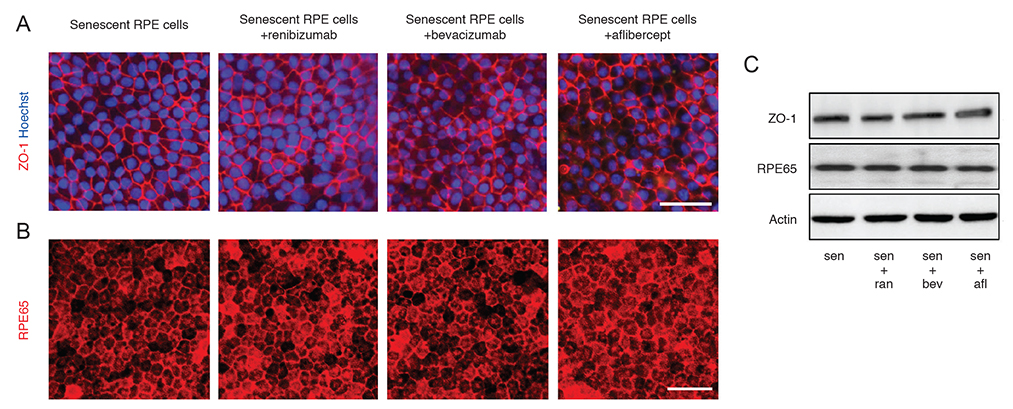
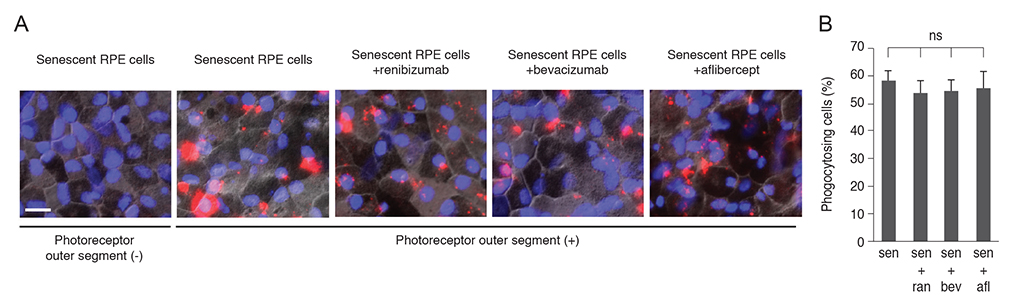
Clinical doses of ranibizumab, bevacizumab, or aflibercept did not decrease the viability or alter proliferation of senescent RPE cells. In addition, the anti-VEGF agents did not induce additional senescence, impair the protein expression of zonula occludens-1 and RPE65, or reduce the phagocytosis capacity of senescent RPE cells.
CONCLUSIONS
Clinical dosages of ranibizumab, bevacizumab, or aflibercept do not induce significant cytotoxicity in senescent RPE cells.
MeSH Terms
Figure
Reference
-
1. Fine SL, Berger JW, Maguire MG, Ho AC. Age-related macular degeneration. N Engl J Med. 2000; 342:483–492.
Article2. Kliffen M, Sharma HS, Mooy CM, et al. Increased expression of angiogenic growth factors in age-related maculopathy. Br J Ophthalmol. 1997; 81:154–162.
Article3. Rakic JM, Lambert V, Devy L, et al. Placental growth factor, a member of the VEGF family, contributes to the development of choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003; 44:3186–3193.
Article4. Kozlowski MR. RPE cell senescence: a key contributor to age-related macular degeneration. Med Hypotheses. 2012; 78:505–510.
Article5. Marazita MC, Dugour A, Marquioni-Ramella MD, et al. Oxidative stress-induced premature senescence dysregulates VEGF and CFH expression in retinal pigment epithelial cells: implications for age-related macular degeneration. Redox Biol. 2016; 7:78–87.
Article6. Mishima K, Handa JT, Aotaki-Keen A, et al. Senescence-associated beta-galactosidase histochemistry for the primate eye. Invest Ophthalmol Vis Sci. 1999; 40:1590–1593.7. Brown DM, Kaiser PK, Michels M, et al. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1432–1444.
Article8. Rosenfeld PJ, Brown DM, Heier JS, et al. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006; 355:1419–1431.
Article9. Yoganathan P, Deramo VA, Lai JC, et al. Visual improvement following intravitreal bevacizumab (Avastin) in exudative age-related macular degeneration. Retina. 2006; 26:994–998.
Article10. Aggio FB, Farah ME, Silva WC, Melo GB. Intravitreal bevacizumab for exudative age-related macular degeneration after multiple treatments. Graefes Arch Clin Exp Ophthalmol. 2007; 245:215–220.
Article11. Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology. 2012; 119:2537–2548.
Article12. Talks JS, Lotery AJ, Ghanchi F, et al. First-year visual acuity outcomes of providing aflibercept according to the VIEW study protocol for age-related macular degeneration. Ophthalmology. 2016; 123:337–343.
Article13. Malik D, Tarek M, Caceres del Carpio J, et al. Safety profiles of anti-VEGF drugs: bevacizumab, ranibizumab, aflibercept and ziv-aflibercept on human retinal pigment epithelium cells in culture. Br J Ophthalmol. 2014; 98 Suppl 1. i11–i16.
Article14. Schnichels S, Hagemann U, Januschowski K, et al. Comparative toxicity and proliferation testing of aflibercept, bevacizumab and ranibizumab on different ocular cells. Br J Ophthalmol. 2013; 97:917–923.
Article15. Ammar DA, Mandava N, Kahook MY. The effects of aflibercept on the viability and metabolism of ocular cells in vitro. Retina. 2013; 33:1056–1061.
Article16. Brar VS, Sharma RK, Murthy RK, Chalam KV. Evaluation of differential toxicity of varying doses of bevacizumab on retinal ganglion cells, retinal pigment epithelial cells, and vascular endothelial growth factor-enriched choroidal endothelial cells. J Ocul Pharmacol Ther. 2009; 25:507–511.
Article17. Klettner A, Tahmaz N, Dithmer M, et al. Effects of aflibercept on primary RPE cells: toxicity, wound healing, uptake and phagocytosis. Br J Ophthalmol. 2014; 98:1448–1452.
Article18. Saenz-de-Viteri M, Fernandez-Robredo P, Hernandez M, et al. Single- and repeated-dose toxicity study of bevacizumab, ranibizumab, and aflibercept in ARPE-19 cells under normal and oxidative stress conditions. Biochem Pharmacol. 2016; 103:129–139.
Article19. Spitzer MS, Wallenfels-Thilo B, Sierra A, et al. Antiproliferative and cytotoxic properties of bevacizumab on different ocular cells. Br J Ophthalmol. 2006; 90:1316–1321.
Article20. Grunwald JE, Pistilli M, Ying GS, et al. Growth of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2015; 122:809–816.
Article21. Grunwald JE, Daniel E, Huang J, et al. Risk of geographic atrophy in the comparison of age-related macular degeneration treatments trials. Ophthalmology. 2014; 121:150–161.22. Zhu Y, Carido M, Meinhardt A, et al. Three-dimensional neuroepithelial culture from human embryonic stem cells and its use for quantitative conversion to retinal pigment epithelium. PLoS One. 2013; 8:e54552.
Article23. Lin H, Clegg DO. Integrin alphavbeta5 participates in the binding of photoreceptor rod outer segments during phagocytosis by cultured human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1998; 39:1703–1712.24. Kuilman T, Michaloglou C, Mooi WJ, Peeper DS. The essence of senescence. Genes Dev. 2010; 24:2463–2479.
Article25. Rufini A, Tucci P, Celardo I, Melino G. Senescence and aging: the critical roles of p53. Oncogene. 2013; 32:5129–5143.
Article26. Harley CB, Futcher AB, Greider CW. Telomeres shorten during ageing of human fibroblasts. Nature. 1990; 345:458–460.
Article27. Zhu H, Belcher M, van der Harst P. Healthy aging and disease: role for telomere biology? Clin Sci (Lond). 2011; 120:427–440.
Article28. Kokkinaki M, Sahibzada N, Golestaneh N. Human induced pluripotent stem-derived retinal pigment epithelium (RPE) cells exhibit ion transport, membrane potential, polarized vascular endothelial growth factor secretion, and gene expression pattern similar to native RPE. Stem Cells. 2011; 29:825–835.
Article29. Ford KM, Saint-Geniez M, Walshe T, et al. Expression and role of VEGF in the adult retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2011; 52:9478–9487.
Article30. Kurihara T, Westenskow PD, Bravo S, et al. Targeted deletion of Vegfa in adult mice induces vision loss. J Clin Invest. 2012; 122:4213–4217.
Article31. Rofagha S, Bhisitkul RB, Boyer DS, et al. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology. 2013; 120:2292–2299.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Intravitreal Aflibercept for Neovascular Age-Related Macular Degeneration Resistant to Bevacizumab and Ranibizumab
- Clinical Changes after Switching from Ranibizumab/Aflibercept to Bevacizumab in Exudative Age-related Macular Degeneration
- Comparison between Aflibercept, Ranibizumab Intravitreal Injection on Neovascular Age-related Macular Degeneration Patients
- Culture of Retinal Pigment Epithelial Cells on Collagen Membrane
- Intravitreal injection of anti-vascular endothelial growth factor for patients with various retinal diseases