Cancer Res Treat.
2018 Jul;50(3):950-955. 10.4143/crt.2017.357.
Utility of the National Lung Screening Trial Criteria for Estimation of Lung Cancer in the Korean Population
- Affiliations
-
- 1Division of Pulmonary and Critical Care Medicine, Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea. mdyspark@gmail.com
- 2Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2417883
- DOI: http://doi.org/10.4143/crt.2017.357
Abstract
- PURPOSE
Screening forlung cancerin high-risk patients using the National Lung Screening Trial (NLST) criteria resulted in a decreased lung cancer-related mortality rate. However, whether these criteria are applicable to the Korean has not been investigated thus far. Therefore, we estimated the utility of the NLST criteria as a screening tool for lung cancer in the Korean population.
MATERIALS AND METHODS
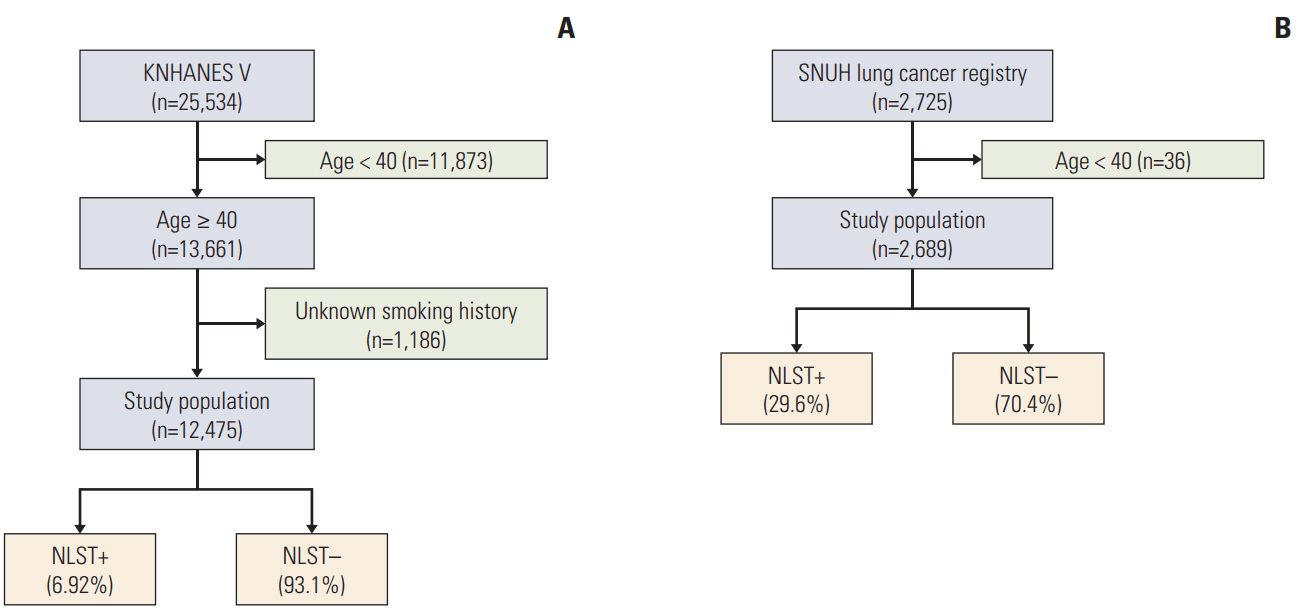
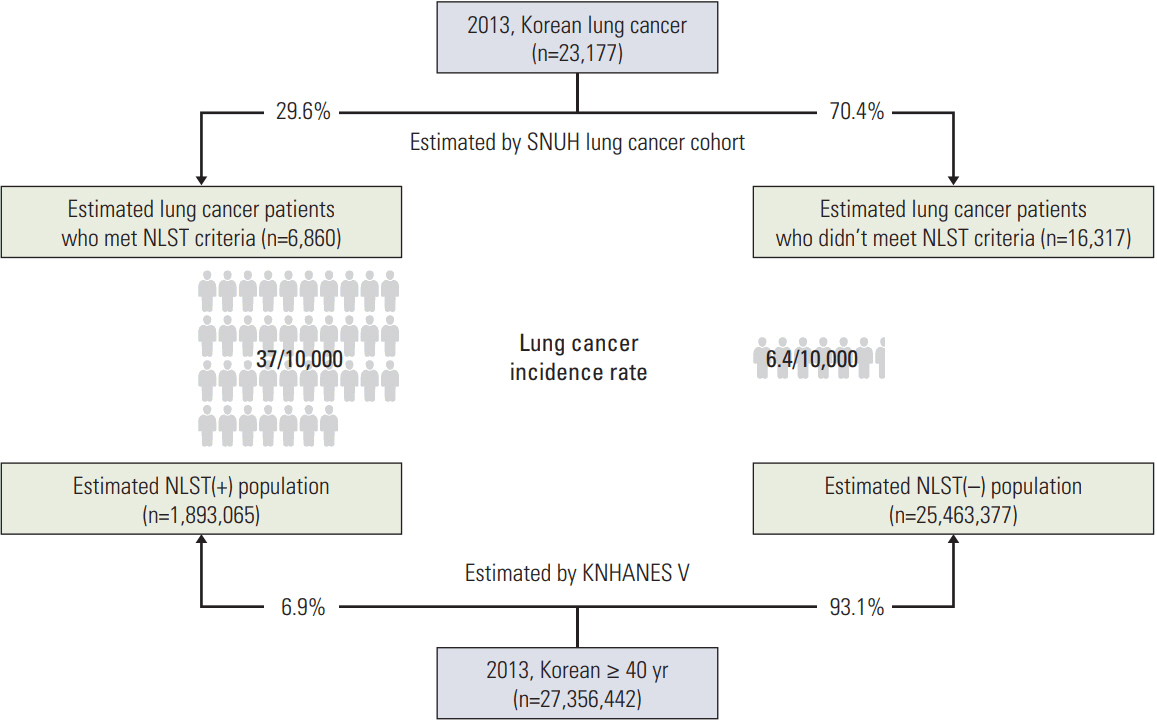
The total number of newly diagnosed lung cancer cases in 2013 was obtained from the Korea National Statistical Office. The proportion of newly diagnosed lung cancer cases that met the NLST criteria was calculated via a retrospective cohort of a tertiary referral hospital. We estimated the nationwide proportion of patients who met the NLST criteria using the 5th Korea National Health and Nutrition Examination Survey conducted during 2010-2012 (KNHANES V).
RESULTS
Using KNHANES V data, we found that approximately 6.92% of the general population of Korea would meet the NLST criteria. In the tertiary referral hospital, 29.6% of the 2,689 newly diagnosed lung cancer patients met the NLST criteria. In 2013, the total number of newly diagnosed lung cancer cases in Korea was 23,177. The estimated nationwide proportions of lung cancer patients who met and did not meet the NLST criteria were 0.37% and 0.06%, respectively, yielding a ratio of 5.78.
CONCLUSION
The NLST criteria demonstrated sound clinical utility for lung cancer screening of high-risk patients in Korea.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
The Incidences and Characteristics of Various Cancers in Patients on Dialysis: a Korean Nationwide Study
Soon Kil Kwon, Joung-Ho Han, Hye-Young Kim, Gilwon Kang, Minseok Kang, Yeonkook J. Kim, Jinsoo Min
J Korean Med Sci. 2019;34(25):. doi: 10.3346/jkms.2019.34.e176.
Reference
-
References
1. Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015; 136:E359–86.
Article2. American Cancer Society. Cancer facts & figures 2017 [Internet]. Atlanta, GA: American Cancer Society;2017. [cited 2017 Oct 1]. Available from: http://www.cancer.org/Research/CancerFactsFigures/index 2017.3. Edwards BK, Brown ML, Wingo PA, Howe HL, Ward E, Ries LA, et al. Annual report to the nation on the status of cancer, 1975-2002, featuring population-based trends in cancer treatment. J Natl Cancer Inst. 2005; 97:1407–27.
Article4. Fontana RS, Sanderson DR, Woolner LB, Taylor WF, Miller WE, Muhm JR, et al. Screening for lung cancer: a critique of the Mayo Lung Project. Cancer. 1991; 67(4 Suppl):1155–64.
Article5. Frame PS. Routine screening for lung cancer?: Maybe someday, but not yet. JAMA. 2000; 284:1980–3.6. National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011; 365:395–409.
Article7. National Lung Screening Trial Research Team, Church TR, Black WC, Aberle DR, Berg CD, Clingan KL, et al. Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med. 2013; 368:1980–91.
Article8. National Comprehensive Cancer Network. Lung cancer screening [Internet]. Fort Washington, PA: National Comprehensive Cancer Network;2014. [cited 2014 Dec 26]. Available from: http://www.nccn.org.9. Moyer VA; U.S. Preventive Services Task Force. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014; 160:330–8.
Article10. Bach PB, Mirkin JN, Oliver TK, Azzoli CG, Berry DA, Brawley OW, et al. Benefits and harms of CT screening for lung cancer: a systematic review. JAMA. 2012; 307:2418–29.11. Jaklitsch MT, Jacobson FL, Austin JH, Field JK, Jett JR, Keshavjee S, et al. The American Association for Thoracic Surgery guidelines for lung cancer screening using low-dose computed tomography scans for lung cancer survivors and other high-risk groups. J Thorac Cardiovasc Surg. 2012; 144:33–8.
Article12. Jang SH, Sheen S, Kim HY, Yim HW, Park BY, Kim JW, et al. The Korean guideline for lung cancer screening. J Korean Med Assoc. 2015; 58:291–301.
Article13. The Ministry of Health and Welfare. Lung cancer screening for high-risk smokers between 55 and 74 years old [Internet]. Goyang: National Cancer Center;c2016. [cited 2017 Oct 1]. Available from: http://www.ncc.re.kr/prBoardView1.ncc?nwsId=2298&searchKey=total&searchValue=&pageNum=7.14. Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC Lung Cancer Staging Project: proposals for the revision of the TNM stage groupings in the forthcoming (seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007; 2:706–14.
Article15. Ministry of Health and Welfare. Cancer incidence, cancer survival rate and prevalence in 2013 [Internet]. Goyang: National Cancer Center;c2015. [cited 2017 Oct 1]. Available from: http://ncc.re.kr/prBoardView1.ncc?nwsId=2188.16. Oh CM, Won YJ, Jung KW, Kong HJ, Cho H, Lee JK, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2013. Cancer Res Treat. 2016; 48:436–50.
Article17. World Health Organization. Global tuberculosis report 2016. Geneva: World Health Organization;2016.18. McKee BJ, Regis SM, McKee AB, Flacke S, Wald C. Performance of ACR Lung-RADS in a Clinical CT Lung Screening Program. J Am Coll Radiol. 2016; 13(2 Suppl):R25–9.
Article19. Ten Haaf K, Jeon J, Tammemagi MC, Han SS, Kong CY, Plevritis SK, et al. Risk prediction models for selection of lung cancer screening candidates: a retrospective validation study. PLoS Med. 2017; 14:e1002277.
Article20. Pinsky PF, Berg CD. Applying the National Lung Screening Trial eligibility criteria to the US population: what percent of the population and of incident lung cancers would be covered? J Med Screen. 2012; 19:154–6.
Article