Intest Res.
2018 Jul;16(3):426-435. 10.5217/ir.2018.16.3.426.
Selective M1 macrophage polarization in granuloma-positive and granuloma-negative Crohn's disease, in comparison to intestinal tuberculosis
- Affiliations
-
- 1Department of Pathology, All India Institute of Medical Sciences, New Delhi, India.
- 2Department of Gastroenterology and Human Nutritions, All India Institute of Medical Sciences, New Delhi, India. vineet.aiims@gmail.com dhiraj@icgeb.res.in
- 3Cellular Immunology Group, International Centre for Genetic Engineering and Biotechnology, New Delhi, India. vineet.aiims@gmail.com dhiraj@icgeb.res.in
- KMID: 2417655
- DOI: http://doi.org/10.5217/ir.2018.16.3.426
Abstract
- BACKGROUND/AIMS
Classical M1 macrophage activation exhibits an inflammatory phenotype while alternative M2 macrophage activation exhibits an anti-inflammatory phenotype. We aimed to determine whether there are discriminant patterns of macrophage polarization in Crohn's disease (CD) and intestinal tuberculosis (iTB).
METHODS
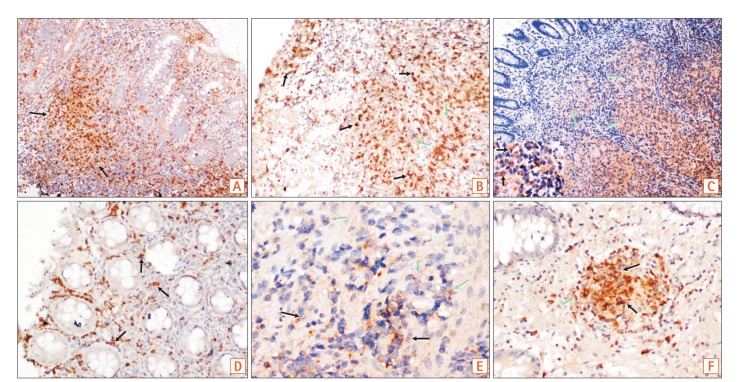
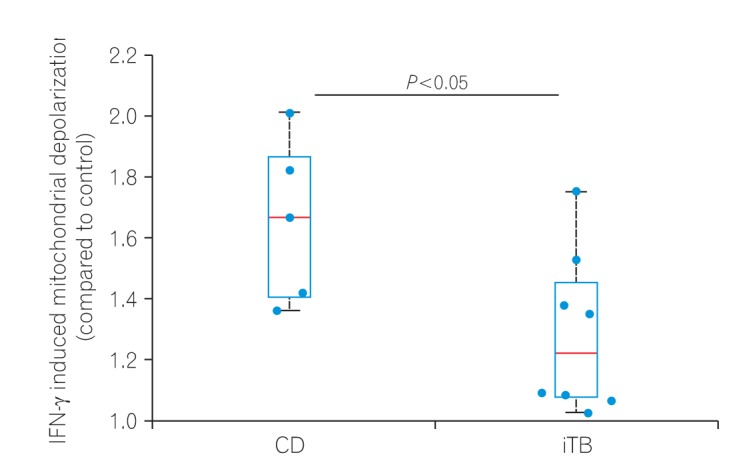
Colonic mucosal biopsies from 29 patients with iTB, 50 with CD, and 19 controls were examined. Dual colored immunohistochemistry was performed for iNOS/CD68 (an M1φ marker) and CD163/CD68 (an M2φ marker), and the ratio of M1φ to M2φ was assessed. To establish the innate nature of macrophage polarization, we analyzed the extent of mitochondrial depolarization, a key marker of inflammatory responses, in monocyte-derived macrophages obtained from CD and iTB patients, following interferon-γ treatment.
RESULTS
M1φ polarization was more prominent in CD biopsies (P=0.002) than in iTB (P=0.2) and control biopsies. In granuloma-positive biopsies, including those in CD, M1φ predominance was significant (P=0.001). In iTB, the densities of M1φ did not differ between granuloma-positive and granuloma-negative biopsies (P=0.1). Interestingly, higher M1φ polarization in CD biopsies correlated with high inflammatory response exhibited by peripheral blood-derived monocytes from these patients.
CONCLUSIONS
Proinflammatory M1φ polarization was more common in colonic mucosa of CD patients, especially in the presence of mucosal granulomas. Further characterization of the innate immune system could help in clarifying the pathology of iTB and CD.
Keyword
MeSH Terms
Figure
Reference
-
1. Makharia GK, Srivastava S, Das P, et al. Clinical, endoscopic, and histological differentiations between Crohn's disease and intestinal tuberculosis. Am J Gastroenterol. 2010; 105:642–651. PMID: 20087333.
Article2. Ahuja V, Tandon RK. Inflammatory bowel disease: the Indian augury. Indian J Gastroenterol. 2012; 31:294–296. PMID: 23150035.
Article3. Pratap Mouli V, Munot K, Ananthakrishnan A, et al. Endoscopic and clinical responses to anti-tubercular therapy can differentiate intestinal tuberculosis from Crohn's disease. Aliment Pharmacol Ther. 2017; 45:27–36. PMID: 27813111.
Article4. Turner K, Genta RM, Lujan G, Robiou C, Sonnenberg A. Significance of the epithelioid granuloma in biopsies of Crohn's colitis. Inflamm Bowel Dis. 2014; 20:2271–2275. PMID: 25208107.
Article5. Freeman HJ. Granuloma-positive Crohn's disease. Can J Gastroenterol. 2007; 21:583–587. PMID: 17853953.
Article6. Neurath MF, Finotto S, Glimcher LH. The role of Th1/Th2 polarization in mucosal immunity. Nat Med. 2002; 8:567–573. PMID: 12042806.
Article7. Maloy KJ, Powrie F. Regulatory T cells in the control of immune pathology. Nat Immunol. 2001; 2:816–822. PMID: 11526392.
Article8. de Jong YP, Abadia-Molina AC, Satoskar AR, et al. Development of chronic colitis is dependent on the cytokine MIF. Nat Immunol. 2001; 2:1061–1066. PMID: 11668338.
Article9. Koboziev I, Karlsson F, Grisham MB. Gut-associated lymphoid tissue, T cell trafficking, and chronic intestinal inflammation. Ann N Y Acad Sci. 2010; 1207(Suppl 1):E86–E93. DOI: 10.1111/j.1749-6632.2010.05711.x. PMID: 20961311.
Article10. Moser M, Murphy KM. Dendritic cell regulation of TH1-TH2 development. Nat Immunol. 2000; 1:199–205. PMID: 10973276.
Article11. Blumberg RS, Saubermann LJ, Strober W. Animal models of mucosal inflammation and their relation to human inflammatory bowel disease. Curr Opin Immunol. 1999; 11:648–656. PMID: 10631550.
Article12. Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014; 6:13. DOI: 10.12703/P6-13. PMID: 24669294.
Article13. Mackaness GB. Cellular resistance to infection. J Exp Med. 1962; 116:381–406. PMID: 14467923.
Article14. Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992; 176:287–292. PMID: 1613462.
Article15. Doyle AG, Herbein G, Montaner LJ, et al. Interleukin-13 alters the activation state of murine macrophages in vitro: comparison with interleukin-4 and interferon-gamma. Eur J Immunol. 1994; 24:1441–1445. PMID: 7911424.
Article16. Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000; 164:6166–6173. PMID: 10843666.
Article17. Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004; 25:677–686. PMID: 15530839.
Article18. Hansen G, Hercus TR, McClure BJ, et al. The structure of the GM-CSF receptor complex reveals a distinct mode of cytokine receptor activation. Cell. 2008; 134:496–507. PMID: 18692472.
Article19. Lacey DC, Achuthan A, Fleetwood AJ, et al. Defining GM-CSF- and macrophage-CSF-dependent macrophage responses by in vitro models. J Immunol. 2012; 188:5752–5765. PMID: 22547697.
Article20. Matta SK, Kumar D. Hypoxia and classical activation limits Mycobacterium tuberculosis survival by Akt-dependent glycolytic shift in macrophages. Cell Death Discov. 2016; 2:16022. DOI: 10.1038/cddiscovery.2016.22. PMID: 27551515.
Article21. Stange EF, Travis SP, Vermeire S, et al. European evidence based consensus on the diagnosis and management of Crohn's disease: definitions and diagnosis. Gut. 2006; 55(Suppl 1):i1–i15. PMID: 16481628.
Article22. Paustian F. Tuberculosis of the intestine. In : Bockus HL, Schaffner F, Berk JE, editors. Bockus gastroenterology. 5th ed. Philadelphia: Saunders;1995. p. 3304.23. Barros MH, Hauck F, Dreyer JH, Kempkes B, Niedobitek G. Macrophage polarisation: an immunohistochemical approach for identifying M1 and M2 macrophages. PLoS One. 2013; 8:e80908. DOI: 10.1371/journal.pone.0080908. PMID: 24260507.
Article24. Buechler C, Ritter M, Orsó E, Langmann T, Klucken J, Schmitz G. Regulation of scavenger receptor CD163 expression in human monocytes and macrophages by pro- and antiinflammatory stimuli. J Leukoc Biol. 2000; 67:97–103. PMID: 10648003.
Article25. Sulahian TH, Högger P, Wahner AE, et al. Human monocytes express CD163, which is upregulated by IL-10 and identical to p155. Cytokine. 2000; 12:1312–1321. PMID: 10975989.
Article26. Isidro RA, Appleyard CB. Colonic macrophage polarization in homeostasis, inflammation, and cancer. Am J Physiol Gastrointest Liver Physiol. 2016; 311:G59–G73. PMID: 27229123.
Article27. Tobal K, Pagliuca A, Bhatt B, Bailey N, Layton DM, Mufti GJ. Mutation of the human FMS gene (M-CSF receptor) in myelodysplastic syndromes and acute myeloid leukemia. Leukemia. 1990; 4:486–489. PMID: 2142747.28. Cossarizza A, Baccarani-Contri M, Kalashnikova G, Franceschi C. A new method for the cytofluorimetric analysis of mitochondrial membrane potential using the J-aggregate forming lipophilic cation 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide (JC-1). Biochem Biophys Res Commun. 1993; 197:40–45. PMID: 8250945.
Article29. Qualls JE, Kaplan AM, van Rooijen N, Cohen DA. Suppression of experimental colitis by intestinal mononuclear phagocytes. J Leukoc Biol. 2006; 80:802–815. PMID: 16888083.
Article30. Tamoutounour S, Henri S, Lelouard H, et al. CD64 distinguishes macrophages from dendritic cells in the gut and reveals the Th1-inducing role of mesenteric lymph node macrophages during colitis. Eur J Immunol. 2012; 42:3150–3166. PMID: 22936024.
Article31. Thiesen S, Janciauskiene S, Uronen-Hansson H, et al. CD14(hi)HLA-DR(dim) macrophages, with a resemblance to classical blood monocytes, dominate inflamed mucosa in Crohn' disease. J Leukoc Biol. 2014; 95:531–541. PMID: 24212097.
Article32. Magnusson MK, Brynjólfsson SF, Dige A, et al. Macrophage and dendritic cell subsets in IBD: ALDH+ cells are reduced in colon tissue of patients with ulcerative colitis regardless of inflammation. Mucosal Immunol. 2016; 9:171–182. PMID: 26080709.
Article33. Kamada N, Hisamatsu T, Okamoto S, et al. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J Clin Invest. 2008; 118:2269–2280. PMID: 18497880.34. Ogino T, Nishimura J, Barman S, et al. Increased Th17-inducing activity of CD14+ CD163 low myeloid cells in intestinal lamina propria of patients with Crohn's disease. Gastroenterology. 2013; 145:1380–1391.e1. PMID: 23993972.
Article35. Lissner D, Schumann M, Batra A, et al. Monocyte and M1 macrophage-induced barrier defect contributes to chronic intestinal inflammation in IBD. Inflamm Bowel Dis. 2015; 21:1297–1305. PMID: 25901973.
Article36. Kühl AA, Erben U, Kredel LI, Siegmund B. Diversity of intestinal macrophages in inflammatory bowel diseases. Front Immunol. 2015; 6:613. DOI: 10.3389/fimmu.2015.00613. PMID: 26697009.
Article37. Schaale K, Brandenburg J, Kispert A, Leitges M, Ehlers S, Reiling N. Wnt6 is expressed in granulomatous lesions of Mycobacterium tuberculosis-infected mice and is involved in macrophage differentiation and proliferation. J Immunol. 2013; 191:5182–5195. PMID: 24123681.
Article38. Lugo-Villarino G, Vérollet C, Maridonneau-Parini I, Neyrolles O. Macrophage polarization: convergence point targeted by mycobacterium tuberculosis and HIV. Front Immunol. 2011; 2:43. DOI: 10.3389/fimmu.2011.00043. PMID: 22566833.
Article39. Jablonski KA, Amici SA, Webb LM, et al. Novel markers to delineate murine M1 and M2 macrophages. PLoS One. 2015; 10:e0145342. DOI: 10.1371/journal.pone.0145342. PMID: 26699615.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Diagnostic Value of Polymerase Chain Reaction in Intestinal Tuberculosis
- A Case of Crohns Disease Misdiagnosed as Intestinal Tuberculosis
- Two Cases of Generalized Granuloma Annulare in Early Childhood
- Macrophage Polarization and Infection
- Paeonol accelerates skin wound healing by regulating macrophage polarization and inflammation in diabetic rats