Intest Res.
2018 Jul;16(3):374-383. 10.5217/ir.2018.16.3.374.
Delineating inflammatory bowel disease through transcriptomic studies: current review of progress and evidence
- Affiliations
-
- 1Department of Physiology, Faculty of Medicine, Universiti Kebangsaan Malaysia Medical Centre, Kuala Lumpur, Malaysia. norfilza@ppukm.ukm.edu.my
- 2Gastroenterology Unit, Department of Medicine, Faculty of Medicine, Universiti Kebangsaan Malaysia Medical Centre, Kuala Lumpur, Malaysia.
- KMID: 2417649
- DOI: http://doi.org/10.5217/ir.2018.16.3.374
Abstract
- Inflammatory bowel disease (IBD), which comprises of Crohn's disease and ulcerative colitis, is an idiopathic relapsing and remitting disease in which the interplay of different environment, microbial, immunological and genetic factors that attribute to the progression of the disease. Numerous studies have been conducted in multiple aspects including clinical, endoscopy and histopathology for the diagnostics and treatment of IBD. However, the molecular mechanism underlying the aetiology and pathogenesis of IBD is still poorly understood. This review tries to critically assess the scientific evidence at the transcriptomic level as it would help in the discovery of RNA molecules in tissues or serum between the healthy and diseased or different IBD subtypes. These molecular signatures could potentially serve as a reliable diagnostic or prognostic biomarker. Researchers have also embarked on the study of transcriptome to be utilized in targeted therapy. We focus on the evaluation and discussion related to the publications reporting the different approaches and techniques used in investigating the transcriptomic changes in IBD with the intention to offer new perspectives to the landscape of the disease.
Keyword
MeSH Terms
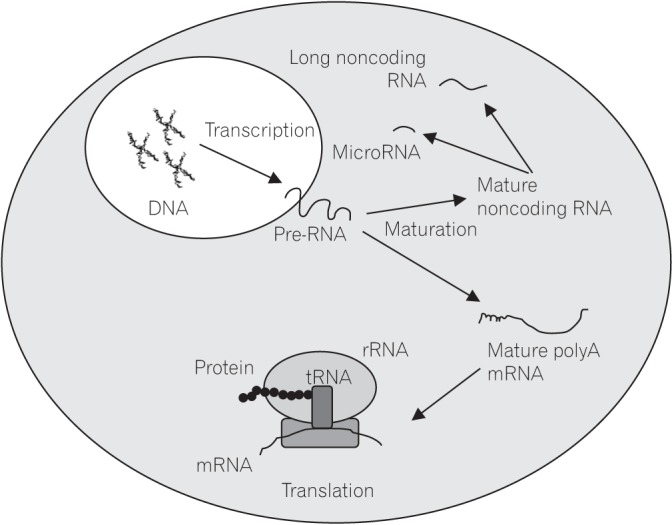
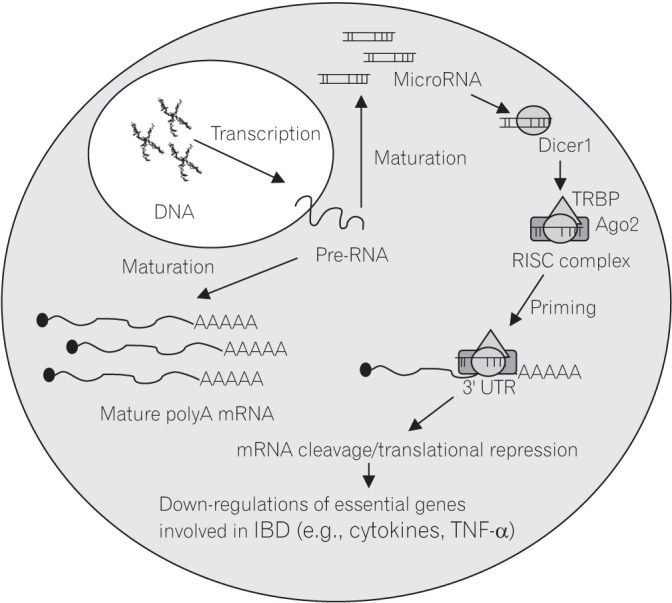
Figure
Reference
-
1. Abraham C, Cho JH. Inflammatory bowel disease. N Engl J Med. 2009; 361:2066–2078. PMID: 19923578.
Article2. Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998; 115:182–205. PMID: 9649475.
Article3. Mirza AH, Berthelsen CH, Seemann SE, et al. Transcriptomic landscape of lncRNAs in inflammatory bowel disease. Genome Med. 2015; 7:39. DOI: 10.1186/s13073-015-0162-2. PMID: 25991924.
Article4. Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010; 28:573–621. PMID: 20192811.
Article5. Zhang YZ, Li YY. Inflammatory bowel disease: pathogenesis. World J Gastroenterol. 2014; 20:91–99. PMID: 24415861.
Article6. Ananthakrishnan AN. Epidemiology and risk factors for IBD. Nat Rev Gastroenterol Hepatol. 2015; 12:205–217. PMID: 25732745.
Article7. Hu PJ. Inflammatory bowel disease in Asia: the challenges and opportunities. Intest Res. 2015; 13:188–190. PMID: 26130991.
Article8. Prideaux L, Kamm MA, De Cruz PP, Chan FK, Ng SC. Inflammatory bowel disease in Asia: a systematic review. J Gastroenterol Hepatol. 2012; 27:1266–1280. PMID: 22497584.
Article9. Ng SC. Epidemiology of inflammatory bowel disease: focus on Asia. Best Pract Res Clin Gastroenterol. 2014; 28:363–372. PMID: 24913377.
Article10. Ali RA. The positive influences of increasing age at diagnosis of inflammatory bowel disease on disease prognostication in Asian perspective. Intest Res. 2015; 13:4–5. PMID: 25691837.
Article11. Ng SC. Emerging trends of inflammatory bowel disease in Asia. Gastroenterol Hepatol (N Y). 2016; 12:193–196. PMID: 27231449.12. Ng SC, Tang W, Ching JY, et al. Incidence and phenotype of inflammatory bowel disease based on results from the Asia-pacific Crohn's and colitis epidemiology study. Gastroenterology. 2013; 145:158–165.e2. PMID: 23583432.
Article13. Kanai T, Matsuoka K, Naganuma M, Hayashi A, Hisamatsu T. Diet, microbiota, and inflammatory bowel disease: lessons from Japanese foods. Korean J Intern Med. 2014; 29:409–415. PMID: 25045286.
Article14. Ng SC. Emerging leadership lecture: inflammatory bowel disease in Asia: emergence of a “Western” disease. J Gastroenterol Hepatol. 2015; 30:440–445. PMID: 25469874.
Article15. Cheon JH. Genetics of inflammatory bowel diseases: a comparison between Western and Eastern perspectives. J Gastroenterol Hepatol. 2013; 28:220–226. PMID: 23189979.
Article16. Söderman J, Berglind L, Almer S. Gene Expression-genotype analysis implicates GSDMA, GSDMB, and LRRC3C as contributors to inflammatory bowel disease susceptibility. Biomed Res Int. 2015; 2015:834805. DOI: 10.1155/2015/834805. PMID: 26484354.17. Xu XR, Liu CQ, Feng BS, Liu ZJ. Dysregulation of mucosal immune response in pathogenesis of inflammatory bowel disease. World J Gastroenterol. 2014; 20:3255–3264. PMID: 24695798.
Article18. Palmieri O, Mazzoccoli G, Bossa F, et al. Systematic analysis of circadian genes using genome-wide cDNA microarrays in the inflammatory bowel disease transcriptome. Chronobiol Int. 2015; 32:903–916. PMID: 26172092.
Article19. Fang K, Grisham MB, Kevil CG. Application of comparative transcriptional genomics to identify molecular targets for pediatric IBD. Front Immunol. 2015; 6:165. DOI: 10.3389/fimmu.2015.00165. PMID: 26085826.
Article20. Planell N, Lozano JJ, Mora-Buch R, et al. Transcriptional analysis of the intestinal mucosa of patients with ulcerative colitis in remission reveals lasting epithelial cell alterations. Gut. 2013; 62:967–976. PMID: 23135761.
Article21. Hong SN, Joung JG, Bae JS, et al. RNA-seq reveals transcriptomic differences in inflamed and noninflamed intestinal mucosa of Crohn's disease patients compared with normal mucosa of healthy controls. Inflamm Bowel Dis. 2017; 23:1098–1108. PMID: 28613228.
Article22. Adams J. Transcriptome: connecting the genome to gene function. Nat Educ. 2008; 1:195.23. Frith MC, Pheasant M, Mattick JS. The amazing complexity of the human transcriptome. Eur J Hum Genet. 2005; 13:894–897. PMID: 15970949.24. Mathew S, Shaabad M, Hussein S, Mira L, Qadri I. The need behind messenger RNA sequencing analysis. Int J Adv Res. 2015; 3:1260–1270.25. Elliott DJ. Illuminating the transcriptome through the genome. Genes (Basel). 2014; 5:235–253. PMID: 24705295.
Article26. Wan Y, Kertesz M, Spitale RC, Segal E, Chang HY. Understanding the transcriptome through RNA structure. Nat Rev Genet. 2011; 12:641–655. PMID: 21850044.
Article27. Peet A, Lieberman M, Marks AD. Marks' basic medical biochemistry (Lieberman, Marks's basic medical biochemistry). 4th ed. Philadelphia: Lippincott Williams & Wilkins;2013.28. Gray NK, Hrabálková L, Scanlon JP, Smith RW. Poly(A)-binding proteins and mRNA localization: who rules the roost? Biochem Soc Trans. 2015; 43:1277–1284. PMID: 26614673.
Article29. Holgersen K, Kutlu B, Fox B, et al. High-resolution gene expression profiling using RNA sequencing in patients with inflammatory bowel disease and in mouse models of colitis. J Crohns Colitis. 2015; 9:492–506. PMID: 25795566.
Article30. Zhang T, Song B, Zhu W, et al. An ileal Crohn's disease gene signature based on whole human genome expression profiles of disease unaffected ileal mucosal biopsies. PLoS One. 2012; 7:e37139. DOI: 10.1371/journal.pone.0037139. PMID: 22606341.
Article31. Wu F, Dong F, Arendovich N, Zhang J, Huang Y, Kwon JH. Divergent influence of microRNA-21 deletion on murine colitis phenotypes. Inflamm Bowel Dis. 2014; 20:1972–1985. PMID: 25222661.
Article32. Li E, Hamm CM, Gulati AS, et al. Inflammatory bowel diseases phenotype, C. difficile and NOD2 genotype are associated with shifts in human ileum associated microbial composition. PLoS One. 2012; 7:e26284. DOI: 10.1371/journal.pone.0026284. PMID: 22719818.
Article33. van Lierop PP, Swagemakers SM, de Bie CI, et al. Gene expression analysis of peripheral cells for subclassification of pediatric inflammatory bowel disease in remission. PLoS One. 2013; 8:e79549. DOI: 10.1371/journal.pone.0079549. PMID: 24260248.
Article34. Zhao Z, Jinde S, Kakiuchi C, Kasai K. Extracellular elevation of adrenomedullin, a gene associated with schizophrenia, suppresses heat shock protein 1A/1B mRNA. Neuroreport. 2016; 27:1312–1316. PMID: 27776076.
Article35. Lee TI, Young RA. Transcriptional regulation and its misregulation in disease. Cell. 2013; 152:1237–1251. PMID: 23498934.
Article36. Xu L, Ma L, Lian J, Yang J, Chen S. Gene expression alterations in inflamed and unaffected colon mucosa from patients with mild inflammatory bowel disease. Mol Med Rep. 2016; 13:2729–2735. PMID: 26861951.
Article37. Costello CM, Mah N, Häsler R, et al. Dissection of the inflammatory bowel disease transcriptome using genome-wide cDNA microarrays. PLoS Med. 2005; 2:e199. DOI: 10.1371/journal.pmed.0020199. PMID: 16107186.
Article38. Karahan G, Sayar N, Gozum G, Bozkurt B, Konu O, Yulug IG. Relative expression of rRNA transcripts and 45S rDNA promoter methylation status are dysregulated in tumors in comparison with matched-normal tissues in breast cancer. Oncol Rep. 2015; 33:3131–3145. PMID: 25962577.
Article39. Porokhovnik LN, Pasekov VP, Egolina NA, et al. Oxidative stress, rRNA genes, and antioxidant enzymes in pathogenesis of schizophrenia and autism: modeling and clinical advices. Zh Obshch Biol. 2013; 74:340–353. PMID: 25438566.40. Peng GH, Fang F, Zheng J, et al. Mitochondrial 12S rRNA variants studies in 456 subjects with hearing loss in seven schools for deaf and mutes in Zhejiang province. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2012; 47:996–1003. PMID: 23328039.41. Torres AG, Batlle E, Ribas de Pouplana L. Role of tRNA modifications in human diseases. Trends Mol Med. 2014; 20:306–314. PMID: 24581449.
Article42. Giegé R, Jühling F, Pütz J, Stadler P, Sauter C, Florentz C. Structure of transfer RNAs: similarity and variability. Wiley Interdiscip Rev RNA. 2012; 3:37–61. PMID: 21957054.
Article43. Thompson DM, Lu C, Green PJ, Parker R. tRNA cleavage is a conserved response to oxidative stress in eukaryotes. RNA. 2008; 14:2095–2103. PMID: 18719243.
Article44. Melançon CE 3rd, Schultz PG. One plasmid selection system for the rapid evolution of aminoacyl-tRNA synthetases. Bioorg Med Chem Lett. 2009; 19:3845–3847. PMID: 19398201.
Article45. Novikova IV, Hennelly SP, Tung CS, Sanbonmatsu KY. Rise of the RNA machines: exploring the structure of long non-coding RNAs. J Mol Biol. 2013; 425:3731–3746. PMID: 23467124.
Article46. Bassett AR, Akhtar A, Barlow DP, et al. Considerations when investigating lncRNA function in vivo. Elife. 2014; 3:e03058. DOI: 10.7554/eLife.03058. PMID: 25124674.
Article47. Shoemaker DD, Schadt EE, Armour CD, et al. Experimental annotation of the human genome using microarray technology. Nature. 2001; 409:922–927. PMID: 11237012.
Article48. Lander ES, Linton LM, Birren B, et al. Initial sequencing and analysis of the human genome. Nature. 2001; 409:860–921. PMID: 11237011.49. Mercer TR, Neph S, Dinger ME, et al. The human mitochondrial transcriptome. Cell. 2011; 146:645–658. PMID: 21854988.
Article50. Kornienko AE, Guenzl PM, Barlow DP, Pauler FM. Gene regulation by the act of long non-coding RNA transcription. BMC Biol. 2013; 11:59. DOI: 10.1186/1741-7007-11-59. PMID: 23721193.
Article51. Muers M. RNA: genome-wide views of long non-coding RNAs. Nat Rev Genet. 2011; 12:742. DOI: 10.1038/nrg3088.52. Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010; 79:351–379. PMID: 20533884.
Article53. Schanen BC, Li X. Transcriptional regulation of mammalian miRNA genes. Genomics. 2011; 97:1–6. PMID: 20977933.
Article54. Chen Y, Stallings RL. Differential patterns of microRNA expression in neuroblastoma are correlated with prognosis, differentiation, and apoptosis. Cancer Res. 2007; 67:976–983. PMID: 17283129.
Article55. Lüningschrör P, Hauser S, Kaltschmidt B, Kaltschmidt C. MicroRNAs in pluripotency, reprogramming and cell fate induction. Biochim Biophys Acta. 2013; 1833:1894–1903. PMID: 23557785.
Article56. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009; 136:215–233. PMID: 19167326.
Article57. Lages E, Ipas H, Guttin A, Nesr H, Berger F, Issartel JP. MicroRNAs: molecular features and role in cancer. Front Biosci (Landmark Ed). 2012; 17:2508–2540. PMID: 22652795.
Article58. Di Stefano V, Zaccagnini G, Capogrossi MC, Martelli F. MicroRNAs as peripheral blood biomarkers of cardiovascular disease. Vascul Pharmacol. 2011; 55:111–118. PMID: 21846509.59. Tijsen AJ, Creemers EE, Moerland PD, et al. MiR423-5p as a circulating biomarker for heart failure. Circ Res. 2010; 106:1035–1039. PMID: 20185794.60. Kim HY, Kwon HY, Thi HT, et al. MicroRNA-132 and microRNA-223 control positive feedback circuit by regulating FOXO3a in inflammatory bowel disease. J Gastroenterol Hepatol. 2016; 31:1727–1735. PMID: 26878986.61. Häsler R, Sheibani-Tezerji R, Sinha A, et al. Uncoupling of mucosal gene regulation, mRNA splicing and adherent microbiota signatures in inflammatory bowel disease. Gut. 2017; 66:2087–2097. PMID: 27694142.62. Palmieri O, Creanza TM, Bossa F, et al. Functional implications of microRNAs in Crohn's disease revealed by integrating microRNA and messenger RNA expression profiling. Int J Mol Sci. 2017; 18:E1580. DOI: 10.3390/ijms18071580. PMID: 28726756.63. Swanson GR, Burgess HJ, Keshavarzian A. Sleep disturbances and inflammatory bowel disease: a potential trigger for disease flare? Expert Rev Clin Immunol. 2011; 7:29–36. PMID: 21162647.64. Pekow JR, Kwon JH. MicroRNAs in inflammatory bowel disease. Inflamm Bowel Dis. 2012; 18:187–193. PMID: 21425211.
Article65. Coskun M, Bjerrum JT, Seidelin JB, Troelsen JT, Olsen J, Nielsen OH. miR-20b, miR-98, miR-125b-1*, and let-7e* as new potential diagnostic biomarkers in ulcerative colitis. World J Gastroenterol. 2013; 19:4289–4299. PMID: 23885139.
Article66. Wang Z, Gerstein M, Snyder M. RNA-Seq: a revolutionary tool for transcriptomics. Nat Rev Genet. 2009; 10:57–63. PMID: 19015660.
Article67. Cardinale CJ, Wei Z, Li J, et al. Transcriptome profiling of human ulcerative colitis mucosa reveals altered expression of pathways enriched in genetic susceptibility loci. PLoS One. 2014; 9:e96153. DOI: 10.1371/journal.pone.0096153. PMID: 24788701.
Article68. Richard H, Schulz MH, Sultan M, et al. Prediction of alternative isoforms from exon expression levels in RNA-Seq experiments. Nucleic Acids Res. 2010; 38:e112. DOI: 10.1093/nar/gkq041. PMID: 20150413.
Article69. Kanaan Z, Rai SN, Eichenberger MR, et al. Differential microRNA expression tracks neoplastic progression in inflammatory bowel disease-associated colorectal cancer. Hum Mutat. 2012; 33:551–560. PMID: 22241525.
Article70. Olaru AV, Yamanaka S, Vazquez C, et al. MicroRNA-224 negatively regulates p21 expression during late neoplastic progression in inflammatory bowel disease. Inflamm Bowel Dis. 2013; 19:471–480. PMID: 23399735.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Precision medicine in inflammatory bowel diseases
- The Pharmacotherapy of Inflammatory Bowel Disease in Child and Adolescence
- The practice of fecal microbiota transplantation in inflammatory bowel disease
- How Should We Do Different Approach to Treat Inflammatory Bowel Disease by Gender Difference?
- Nutritional Support in Patients with Inflammatory Bowel Diseases