J Korean Neurosurg Soc.
2016 Nov;59(6):647-649. 10.3340/jkns.2016.59.6.647.
Foreign Body Reaction after Implantation of a Device for Intervertebral Assisted Motion
- Affiliations
-
- 1Department of Orthopaedic Surgery, Jeju National University Hospital, School of Medicine, Jeju National University, Jeju, Korea.
- 2Department of Orthopaedic Surgery, Seoul St. Mary's Hospital, College of Medicine, The Catholic University of Korea, Seoul, Korea. kyh@catholic.ac.kr
- KMID: 2417350
- DOI: http://doi.org/10.3340/jkns.2016.59.6.647
Abstract
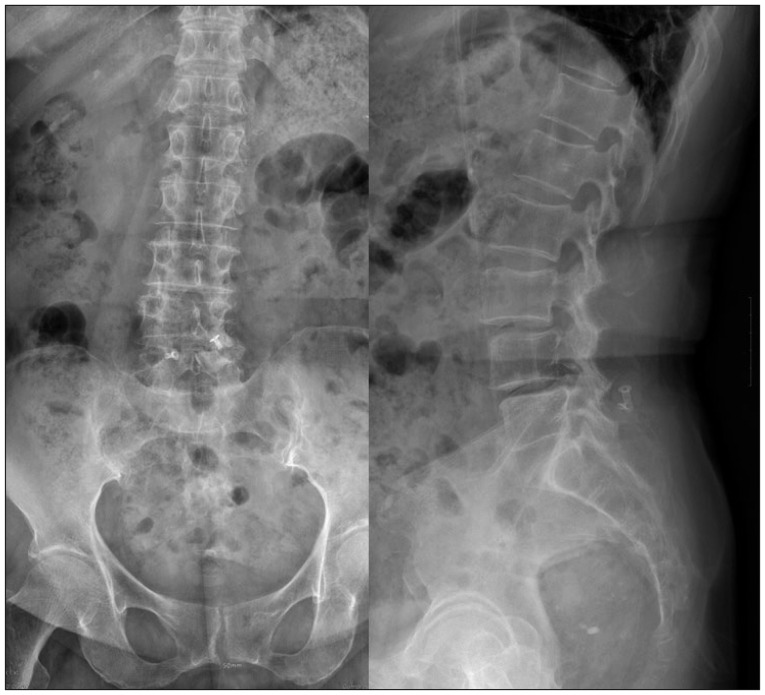
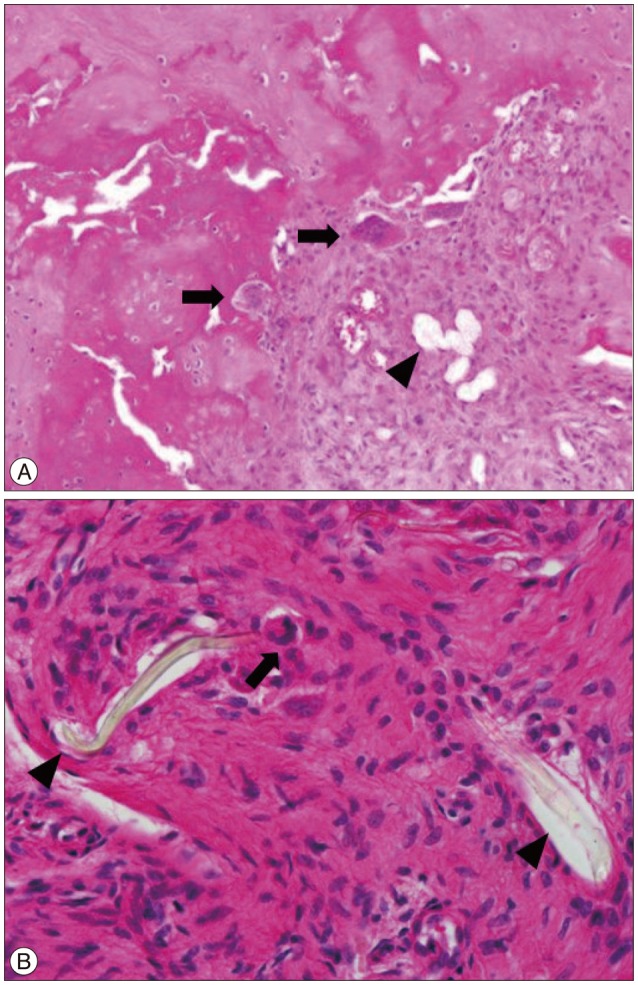
- The device for intervertebral assisted motion (DIAM) is a dynamic implant that consists of a silicone bumper enveloped by a polyethylene terephthalate (PET) fiber sack. Silicone and PET were used because of their biological inertness, but repetitive motion of the spine can cause wear on the implant nonetheless. The purpose of this study is to report a case of foreign body reaction (FBR) against a DIAM. A 72-year-old female patient presented with lower back pain and both legs radiating pain. She had undergone DIAM implantation at L4-5 for spinal stenosis 5 years previously. The intervertebral disc space of L4-5, where the DIAM was inserted, had collapsed and degenerative scoliosis had developed due to left-side collapse. MRI showed L3-4 thecal sac compression and left L4-5 foraminal stenosis. The patient underwent removal of the DIAM and instrumented fusion from L3 to L5. During surgery, fluid and granulation tissue were evident around the DIAM. Histopathology showed scattered wear debris from the DIAM causing chronic inflammation due to the resulting FBR. A FBR due to wear debris of a DIAM can induce a hypersensitivity reaction and bone resorption around the implant, causing it to loosen.
Keyword
MeSH Terms
-
Aged
Bone Resorption
Constriction, Pathologic
Female
Foreign Bodies*
Foreign-Body Reaction*
Granulation Tissue
Humans
Hypersensitivity
Inflammation
Intervertebral Disc
Leg
Low Back Pain
Magnetic Resonance Imaging
Polyethylene Terephthalates
Scoliosis
Silicon
Silicones
Spinal Stenosis
Spine
Polyethylene Terephthalates
Silicon
Silicones
Figure
Reference
-
1. Akhaddar A, Boulahroud O, Naama O, Al-Bouzidi A, Boucetta M. Paraspinal textiloma after posterior lumbar surgery : a wolf in sheep's clothing. World Neurosurg. 2012; 77:375–380. PMID: 22120328.
Article2. Anderson JM. Inflammatory response to implants. ASAIO Trans. 1988; 34:101–107. PMID: 3285869.
Article3. Anderson JM. Multinucleated giant cells. Curr Opin Hematol. 2000; 7:40–47. PMID: 10608503.
Article4. Cunningham BW, Hallab NJ, Hu N, McAfee PC. Epidural application of spinal instrumentation particulate wear debris : a comprehensive evaluation of neurotoxicity using an in vivo animal model. J Neurosurg Spine. 2013; 19:336–350. PMID: 23808583.
Article5. Daniel J, Holland J, Quigley L, Sprague S, Bhandari M. Pseudotumors associated with total hip arthroplasty. J Bone Joint Surg Am. 2012; 94:86–93. PMID: 22218386.
Article6. Ha KY, Seo JY, Kwon SE, Son IN, Kim KW, Kim YH. Posterior dynamic stabilization in the treatment of degenerative lumbar stenosis : validity of its rationale. J Neurosurg Spine. 2013; 18:24–31. PMID: 23140127.
Article7. Jerosch J, Moursi MG. Foreign body reaction due to polyethylene's wear after implantation of an interspinal segment. Arch Orthop Trauma Surg. 2008; 128:1–4. PMID: 17713775.
Article8. Kim DH, Albert TJ. Interspinous process spacers. J Am Acad Orthop Surg. 2007; 15:200–207. PMID: 17426291.
Article9. Potter EH, Rohrich RJ, Bolden KM. The role of silicone granulomas in recurrent capsular contracture : a review of the literature and an approach to management. Plast Reconstr Surg. 2013; 131:888e–895e.10. Rodriguez A, Meyerson H, Anderson JM. Quantitative in vivo cytokine analysis at synthetic biomaterial implant sites. J Biomed Mater Res A. 2009; 89:152–159. PMID: 18431759.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- An Intradiscal Granuloma Due to a Retained Wooden Foreign Body
- A Case of Foreign Body Granuloma after Squalene Injection by Non-dermatologists
- A Stainless Steel Foreign body in the Knee Joint With Synovitis: A Case Report
- Treatment of inflammatory foreign body reaction in tattooed eyebrows by dermabrasion
- A Case of Foreign Body Granulomatous Reaction to a Red Lip Cosmetic Tattoo Successfully Treated with Carbon Dioxide Laser