Obstet Gynecol Sci.
2018 May;61(3):344-351. 10.5468/ogs.2018.61.3.344.
Preoperative serum levels of cancer antigen 125 and carcinoembryonic antigen ratio can improve differentiation between mucinous ovarian carcinoma and other epithelial ovarian carcinomas
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea. CHAYDB@yuhs.ac
- KMID: 2416122
- DOI: http://doi.org/10.5468/ogs.2018.61.3.344
Abstract
OBJECTIVE
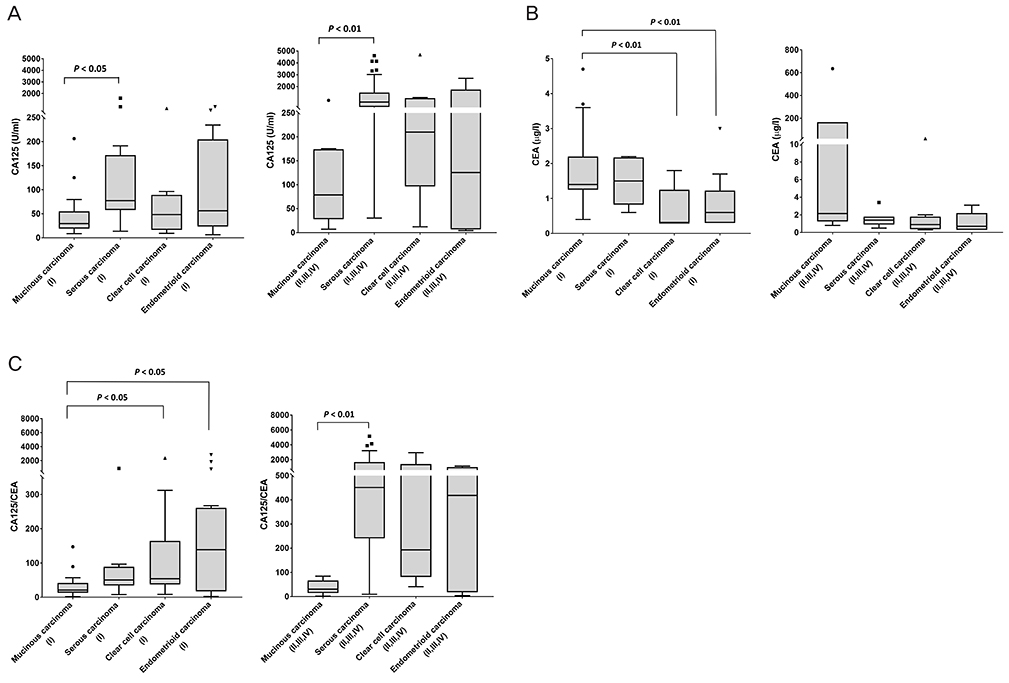
The main aim of this study was to evaluate cancer antigen 125 (CA125)/carcinoembryonic antigen (CEA) ratio (CCR), as a reliable marker to differentiate ovarian mucinous carcinoma from other epithelial ovarian carcinomas (EOCs), namely serous, clear cell, and endometrioid carcinomas.
METHODS
Female patients suffering from different kinds of EOCs whom were subjected to elective surgery at the Gangnam Severance Hospital between January 2008 and December 2016, were included in this study. The serum levels of CA125 and CEA were assayed using commercially available kits per the manufacturer's instructions.
RESULTS
The CCR in mucinous carcinoma (mean 32.1) was significantly lower than that of clear cell (mean 235.0) and endometrioid carcinoma (mean 427.0) in stage I (all P < 0.05). In stage II-IV, CCR in mucinous carcinoma (mean 37.6) was significantly lower than that of serous carcinoma (mean 148.0) (P < 0.01). The sensitivity and specificity of CCR in detecting mucinous carcinoma from other types of EOC was 75.0% and 77.5%, respectively in stage I and 100.0% and 84.4%, respectively in stage II-IV (both cut-off value < 90.7).
CONCLUSION
The present results suggest that pretreatment CCR might provide higher specificity and clinically relevant information as a criterion for the differentiation between ovarian mucinous carcinoma and other types of EOC.
MeSH Terms
Figure
Reference
-
1. Prat J. Ovarian carcinomas: five distinct diseases with different origins, genetic alterations, and clinicopathological features. Virchows Arch. 2012; 460:237–249.
Article2. Sturgeon CM, Duffy MJ, Stenman UH, Lilja H, Brunner N, Chan DW, et al. National Academy of Clinical Biochemistry laboratory medicine practice guidelines for use of tumor markers in testicular, prostate, colorectal, breast, and ovarian cancers. Clin Chem. 2008; 54:e11–e79.
Article3. Badgwell D, Bast RC Jr. Early detection of ovarian cancer. Dis Markers. 2007; 23:397–410.
Article4. Moore RG, Miller MC, Steinhoff MM, Skates SJ, Lu KH, Lambert-Messerlian G, et al. Serum HE4 levels are less frequently elevated than CA125 in women with benign gynecologic disorders. Am J Obstet Gynecol. 2012; 206:351.e1–351.e8.
Article5. Rustin GJ, Nelstrop AE, McClean P, Brady MF, McGuire WP, Hoskins WJ, et al. Defining response of ovarian carcinoma to initial chemotherapy according to serum CA 125. J Clin Oncol. 1996; 14:1545–1551.
Article6. Rustin GJ, Nelstrop AE, Bentzen SM, Bond SJ, McClean P. Selection of active drugs for ovarian cancer based on CA-125 and standard response rates in phase II trials. J Clin Oncol. 2000; 18:1733–1739.
Article7. Yamashita K, Watanabe M. Clinical significance of tumor markers and an emerging perspective on colorectal cancer. Cancer Sci. 2009; 100:195–199.
Article8. Chou YY, Jeng YM, Kao HL, Chen T, Mao TL, Lin MC. Differentiation of ovarian mucinous carcinoma and metastatic colorectal adenocarcinoma by immunostaining with beta-catenin. Histopathology. 2003; 43:151–156.9. Lagendijk JH, Mullink H, Van Diest PJ, Meijer GA, Meijer CJ. Tracing the origin of adenocarcinomas with unknown primary using immunohistochemistry: differential diagnosis between colonic and ovarian carcinomas as primary sites. Hum Pathol. 1998; 29:491–497.
Article10. Raspollini MR, Amunni G, Villanucci A, Baroni G, Taddei A, Taddei GL. Utility of CDX-2 in distinguishing between primary and secondary (intestinal) mucinous ovarian carcinoma: an immunohistochemical comparison of 43 cases. Appl Immunohistochem Mol Morphol. 2004; 12:127–131.11. Sorensen SS, Mosgaard BJ. Combination of cancer antigen 125 and carcinoembryonic antigen can improve ovarian cancer diagnosis. Dan Med Bull. 2011; 58:A4331.12. Changes in definitions of clinical staging for carcinoma of the cervix and ovary: International Federation of Gynecology and Obstetrics. Am J Obstet Gynecol. 1987; 156:263–264.13. Bast RC Jr, Feeney M, Lazarus H, Nadler LM, Colvin RB, Knapp RC. Reactivity of a monoclonal antibody with human ovarian carcinoma. J Clin Invest. 1981; 68:1331–1337.
Article14. Bast RC Jr, Klug TL, St John E, Jenison E, Niloff JM, Lazarus H, et al. A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med. 1983; 309:883–887.
Article15. Bast RC Jr, Klug TL, Schaetzl E, Lavin P, Niloff JM, Greber TF, et al. Monitoring human ovarian carcinoma with a combination of CA 125, CA 19-9, and carcinoembryonic antigen. Am J Obstet Gynecol. 1984; 149:553–559.
Article16. Einhorn N, Bast RC Jr, Knapp RC, Tjernberg B, Zurawski VR Jr. Preoperative evaluation of serum CA 125 levels in patients with primary epithelial ovarian cancer. Obstet Gynecol. 1986; 67:414–416.17. Jacobs I, Bast RC Jr. The CA 125 tumour-associated antigen: a review of the literature. Hum Reprod. 1989; 4:1–12.
Article18. Hogdall EV, Christensen L, Kjaer SK, Blaakaer J, Kjaerbye-Thygesen A, Gayther S, et al. CA125 expression pattern, prognosis and correlation with serum CA125 in ovarian tumor patients. From The Danish “MALOVA” Ovarian Cancer Study. Gynecol Oncol. 2007; 104:508–515.19. Kenemans P, Yedema CA, Bon GG, von Mensdorff-Pouilly S. CA 125 in gynecological pathology--a review. Eur J Obstet Gynecol Reprod Biol. 1993; 49:115–124.20. de Bruijn HW, van der Zee AG, Aalders JG. The value of cancer antigen 125 (CA 125) during treatment and follow-up of patients with ovarian cancer. Curr Opin Obstet Gynecol. 1997; 9:8–13.
Article21. Chen DX, Schwartz PE, Li XG, Yang Z. Evaluation of CA 125 levels in differentiating malignant from benign tumors in patients with pelvic masses. Obstet Gynecol. 1988; 72:23–27.22. Patsner B, Mann WJ. The value of preoperative serum CA 125 levels in patients with a pelvic mass. Am J Obstet Gynecol. 1988; 159:873–876.
Article23. Vasilev SA, Schlaerth JB, Campeau J, Morrow CP. Serum CA 125 levels in preoperative evaluation of pelvic masses. Obstet Gynecol. 1988; 71:751–756.24. Smith LH, Oi RH. Detection of malignant ovarian neoplasms: a review of the literature. II. Laboratory detection. Obstet Gynecol Surv. 1984; 39:329–345.25. Fenoglio CM, Ferenczy A, Richart RM. Mucinous tumors of the ovary. Ultrastructural studies of mucinous cystadenomas with histogenetic considerations. Cancer. 1975; 36:1709–1722.26. Gall SA, Walling J, Pearl J. Demonstration of tumor-associated antigens in human gynecologic malignancies. Am J Obstet Gynecol. 1973; 115:387–393.
Article27. Hogdall EV, Christensen L, Kjaer SK, Blaakaer J, Jarle Christensen I, Gayther S, et al. Protein expression levels of carcinoembryonic antigen (CEA) in Danish ovarian cancer patients: from the Danish 'MALOVA'ovarian cancer study. Pathology. 2008; 40:487–492.28. Tholander B, Taube A, Lindgren A, Sjoberg O, Stendahl U, Tamsen L. Pretreatment serum levels of CA-125, carcinoembryonic antigen, tissue polypeptide antigen, and placental alkaline phosphatase in patients with ovarian carcinoma: influence of histological type, grade of differentiation, and clinical stage of disease. Gynecol Oncol. 1990; 39:26–33.
Article29. Yabushita H, Masuda T, Ogawa A, Noguchi M, Ishihara M. Combination assay of CA125, TPA, IAP, CEA, and ferritin in serum for ovarian cancer. Gynecol Oncol. 1988; 29:66–75.
Article30. Wu JT, Miya T, Knight JA, Knight DP. Improved specificity of the CA 125 enzyme immunoassay for ovarian carcinomas by use of the ratio of CA 125 to carcinoembryonic antigen. Clin Chem. 1988; 34:1853–1857.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Diagnostic Significance of Serum Tumor Markers in Paitents with Ovarian Tumors
- The clinical value of serum TPS and CA 125 in the diagnosis of epithelial ovarian cancer
- A clinical evaluation of CA 125 antigen values in patients of ovarian cancer
- The Usefulness of CDX-2 for Differentiating Primary and Metastatic Ovarian Carcinoma: An Immunohistochemical Study Using a Tissue Microarray
- The Clinical Significance of Carcinoembryonic Antigen and CA72-4 Assays of Peritoneal Fluid in Colorectal Carcinomas