Immune Netw.
2018 Jun;18(3):e21. 10.4110/in.2018.18.e21.
N-terminal Domain of the Spike Protein of Porcine Epidemic Diarrhea Virus as a New Candidate Molecule for a Mucosal Vaccine
- Affiliations
-
- 1Department of Molecular Biology and the Institute for Molecular Biology and Genetics, Chonbuk National University, Jeonju 54896, Korea. yongsuk@jbnu.ac.kr
- 2Department of Bioactive Material Sciences and Research Center of Bioactive Materials, Chonbuk National University, Jeonju 54896, Korea.
- 3Department of Oral Microbiology and Institute of Oral Bioscience, Chonbuk National University, Jeonju 54896, Korea.
- KMID: 2414556
- DOI: http://doi.org/10.4110/in.2018.18.e21
Abstract
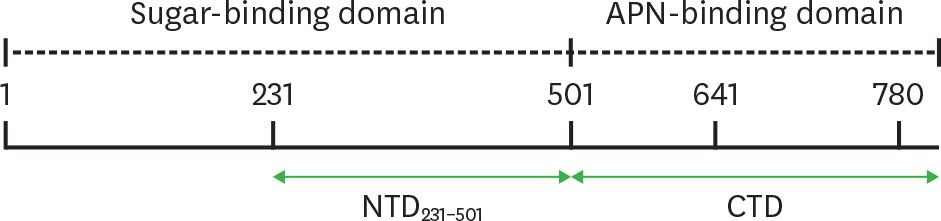
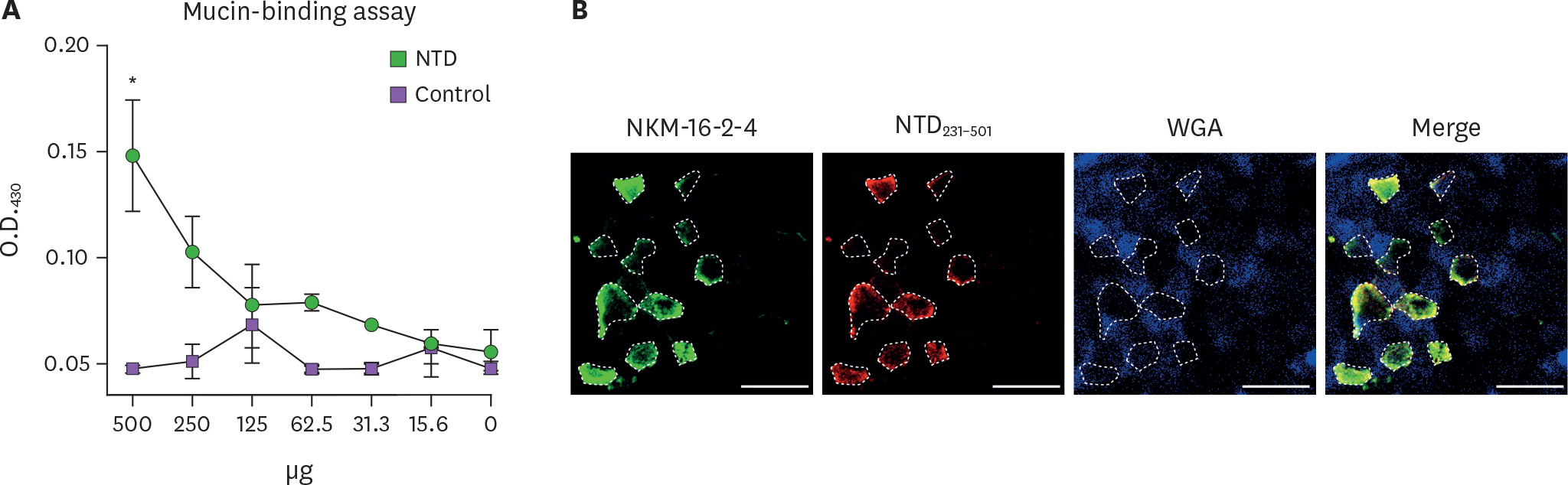
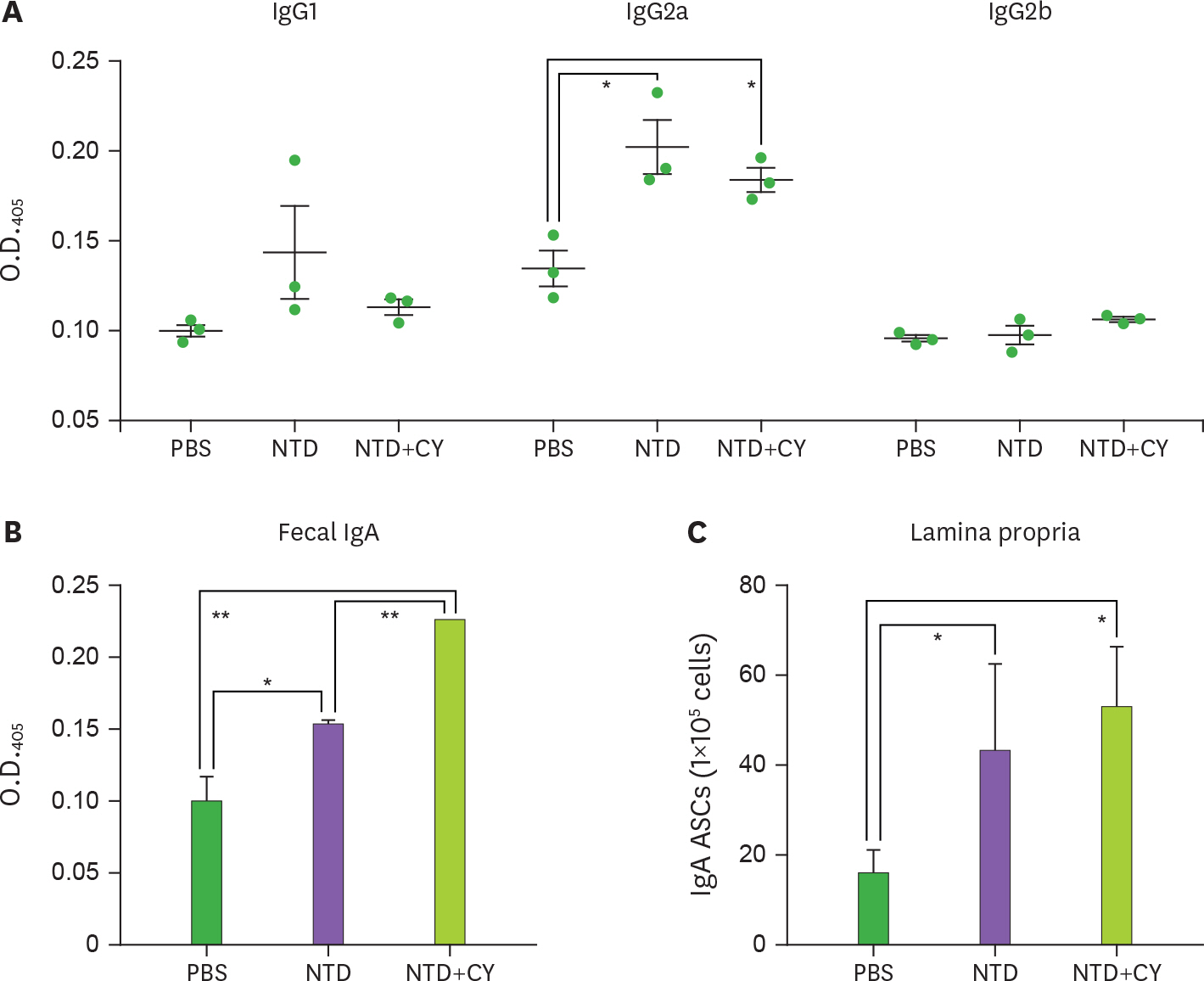
- Porcine epidemic diarrhea virus (PEDV) is a contagious coronavirus infecting pigs that leads to significant economic losses in the swine industry. Given that PEDV infection occurs in gut epithelial cells mainly via the fecal-oral route, induction of PEDV-specific immune responses in the mucosal compartment is required for protective immunity against viral infection. However, an effective mucosal vaccine against the currently prevalent PEDV strain is not available. In this study, we demonstrated that the N-terminal domain (NTD) of the spike (S) protein of PEDV represents a new vaccine candidate molecule to be applied via the mucosal route. We first established an Escherichia coli expression system producing the partial NTD (NTD231-501) of the PEDV S protein. Orally administered NTD231-501 protein specifically interacted with the apical area of M cells in the follicle-associated epithelium of Peyer's patch. Additionally, the NTD protein induced antigen-specific immune responses in both the systemic and mucosal immune compartments when administered orally. Collectively, we propose the NTD of the PEDV S protein to be a candidate mucosal vaccine molecule.
MeSH Terms
Figure
Reference
-
References
1. Wood EN. An apparently new syndrome of porcine epidemic diarrhoea. Vet Rec. 1977; 100:243–244.
Article2. Pensaert MB, de Bouck P. A new coronavirus-like particle associated with diarrhea in swine. Arch Virol. 1978; 58:243–247.
Article3. Deng F, Ye G, Liu Q, Navid MT, Zhong X, Li Y, Wan C, Xiao S, He Q, Fu ZF, et al. Identification and comparison of receptor binding characteristics of the spike protein of two porcine epidemic diarrhea virus strains. Viruses. 2016; 8:55.
Article4. Kocherhans R, Bridgen A, Ackermann M, Tobler K. Completion of the porcine epidemic diarrhoea coronavirus (PEDV) genome sequence. Virus Genes. 2001; 23:137–144.5. Li BX, Ge JW, Li YJ. Porcine aminopeptidase N is a functional receptor for the PEDV coronavirus. Virology. 2007; 365:166–172.
Article6. Alonso C, Goede DP, Morrison RB, Davies PR, Rovira A, Marthaler DG, Torremorell M. Evidence of infectivity of airborne porcine epidemic diarrhea virus and detection of airborne viral RNA at long distances from infected herds. Vet Res (Faisalabad). 2014; 45:73.
Article7. de Arriba ML, Carvajal A, Pozo J, Rubio P. Mucosal and systemic isotype-specific antibody responses and protection in conventional pigs exposed to virulent or attenuated porcine epidemic diarrhoea virus. Vet Immunol Immunopathol. 2002; 85:85–97.
Article8. Liu C, Tang J, Ma Y, Liang X, Yang Y, Peng G, Qi Q, Jiang S, Li J, Du L, et al. Receptor usage and cell entry of porcine epidemic diarrhea coronavirus. J Virol. 2015; 89:6121–6125.
Article9. Brandtzaeg P, Kiyono H, Pabst R, Russell MW. Terminology: nomenclature of mucosa-associated lymphoid tissue. Mucosal Immunol. 2008; 1:31–37.
Article10. Brandtzaeg P, Pabst R. Let's go mucosal: communication on slippery ground. Trends Immunol. 2004; 25:570–577.
Article11. Kucharzik T, Lügering N, Rautenberg K, Lügering A, Schmidt MA, Stoll R, Domschke W. Role of M cells in intestinal barrier function. Ann N Y Acad Sci. 2000; 915:171–183.
Article12. Strugnell RA, Wijburg OL. The role of secretory antibodies in infection immunity. Nat Rev Microbiol. 2010; 8:656–667.
Article13. Frey A, Neutra MR. Targeting of mucosal vaccines to Peyer's patch M cells. Behring Inst Mitt. 1997; 98:376–389.14. Hase K, Kawano K, Nochi T, Pontes GS, Fukuda S, Ebisawa M, Kadokura K, Tobe T, Fujimura Y, Kawano S, et al. Uptake through glycoprotein 2 of FimH+ bacteria by M cells initiates mucosal immune response. Nature. 2009; 462:226–230.15. Kim SH, Jung DI, Yang IY, Kim J, Lee KY, Nochi T, Kiyono H, Jang YS. M cells expressing the complement C5a receptor are efficient targets for mucosal vaccine delivery. Eur J Immunol. 2011; 41:3219–3229.
Article16. Nochi T, Yuki Y, Matsumura A, Mejima M, Terahara K, Kim DY, Fukuyama S, Iwatsuki-Horimoto K, Kawaoka Y, Kohda T, et al. A novel M cell-specific carbohydrate-targeted mucosal vaccine effectively induces antigen-specific immune responses. J Exp Med. 2007; 204:2789–2796.
Article17. Li X, Chen H. Evaluation of the porcine gastric mucin binding assay for high-pressure-inactivation studies using murine norovirus and tulane virus. Appl Environ Microbiol. 2015; 81:515–521.
Article18. Chung HC, Lee JH, Nguyen VG, Huynh TM, Lee GE, Moon HJ, Park SJ, Kim HK, Park BK. New emergence pattern with variant porcine epidemic diarrhea viruses, South Korea, 2012–2015. Virus Res. 2016; 226:14–19.
Article19. Chung HC, Nguyen VG, Moon HJ, Lee JH, Park SJ, Lee GE, Kim HK, Noh YS, Lee CH, Goede D, et al. Isolation of porcine epidemic diarrhea virus during outbreaks in South Korea, 2013–2014. Emerg Infect Dis. 2015; 21:2238–2240.
Article20. Kim SH, Lee KY, Jang YS. Mucosal immune system and M cell-targeting strategies for oral mucosal vaccination. Immune Netw. 2012; 12:165–175.
Article21. Gracie JA, Bradley JA. Interleukin-12 induces interferon-gamma-dependent switching of IgG alloantibody subclass. Eur J Immunol. 1996; 26:1217–1221.22. Gerdts V, Zakhartchouk A. Vaccines for porcine epidemic diarrhea virus and other swine coronaviruses. Vet Microbiol. 2017; 206:45–51.
Article23. Harper S, Speicher DW. Expression and purification of GST fusion proteins. Curr Protoc Protein Sci. 2008. ;Chapter 6: Unit 6.6.
Article24. Li W, Luo R, He Q, van Kuppeveld FJ, Rottier PJ, Bosch BJ. Aminopeptidase N is not required for porcine epidemic diarrhea virus cell entry. Virus Res. 2017; 235:6–13.
Article25. Lycke N. Recent progress in mucosal vaccine development: potential and limitations. Nat Rev Immunol. 2012; 12:592–605.
Article26. Nochi T, Yuki Y, Katakai Y, Shibata H, Tokuhara D, Mejima M, Kurokawa S, Takahashi Y, Nakanishi U, Ono F, et al. A rice-based oral cholera vaccine induces macaque-specific systemic neutralizing antibodies but does not influence pre-existing intestinal immunity. J Immunol. 2009; 183:6538–6544.
Article27. Huber VC, McKeon RM, Brackin MN, Miller LA, Keating R, Brown SA, Makarova N, Perez DR, Macdonald GH, McCullers JA. Distinct contributions of vaccine-induced immunoglobulin G1 (IgG1) and IgG2a antibodies to protective immunity against influenza. Clin Vaccine Immunol. 2006; 13:981–990.
Article28. Huber VC, Lynch JM, Bucher DJ, Le J, Metzger DW. Fc receptor-mediated phagocytosis makes a significant contribution to clearance of influenza virus infections. J Immunol. 2001; 166:7381–7388.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Recent vaccine technology in industrial animals
- Isolation, characterization and neutralizing activity of porcine epidemic diarrhea viruses from Vietnam
- Sequence analysis of the spike gene of Porcine epidemic diarrhea virus isolated from South China during 2011–2015
- Enhancement of antigen-specific humoral immune responses and protein solubility through conjugation of bacterial flagellin, Vibrio vulnificus FlaB, to the N-terminus of porcine epidemic diarrhea virus surface protein antigen S0
- Porcine epidemic diarrhea viruses from Vietnam: isolation, characterization, and neutralizing activity