Transl Clin Pharmacol.
2018 Jun;26(2):73-78. 10.12793/tcp.2018.26.2.73.
Pharmacokinetics comparison of solifenacin tartrate and solifenacin succinate: a randomized, open-label, single-dose, 2-way crossover study in healthy male volunteers
- Affiliations
-
- 1Center for Clinical Pharmacology and Biomedical Research Institute, Chonbuk National University Hospital, Jeonju 54907, Republic of Korea. mgkim@jbnu.ac.kr
- 2Department of Pharmacology, School of Medicine, Chonbuk National University, Jeonju 54907, Republic of Korea.
- 3Hanmi Pharmaceuticals Co., Ltd., Seoul 05545, Republic of Korea.
- KMID: 2413830
- DOI: http://doi.org/10.12793/tcp.2018.26.2.73
Abstract
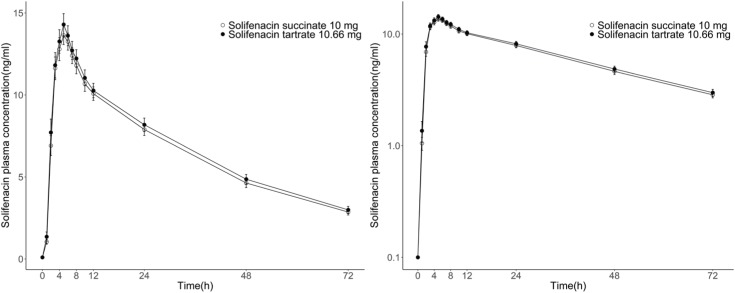
- Solifenacin is a muscarinic antagonist indicated for the treatment of overactive bladder with symptoms. Solifenacin tartrate is a newly developed salt formulation of solifenacin. This study compared the pharmacokinetic and safety properties after single-dose administration of solifenacin tartrate (test formulation) and solifenacin succinate (reference formulation) in healthy male volunteers. A total of 36 subjects were enrolled in this randomized, open-label, single-dose, two-way crossover study. During each treatment period, subjects received the test formulation or reference formulation. Plasma samples were collected at pre-dose and at 1, 2, 3, 4, 5, 6, 7, 8, 10, 12, 24, 48 and 72 hours post-dose. Safety was assessed by adverse events, physical examinations, laboratory assessments, 12-lead electrocardiograms, and vital signs. Thirty-three subjects completed the study and were included in the pharmacokinetic analysis. The mean (standard deviation) values of AUC(last) for the test and reference formulations were 486.98 (138.47) and 469.07 (128.29) h·ng/mL, respectively. The mean (standard deviation) values of C(max) for the test and reference formulations were 14.66 (3.85) and 14.10 (3.37) ng/mL, respectively. The 90% confidence intervals for AUC(last) and C(max) were 0.9702 to 1.1097 and 0.9779 to 1.0993, respectively. All adverse events were mild or moderate, and there were no serious adverse events. The pharmacokinetic properties of solifenacin tartrate were similar to those of solifenacin succinate and met the acceptance criteria for bioequivalence. Both formulations were safe, and no significant difference was observed in the safety assessments of the formulations.
Keyword
MeSH Terms
Figure
Reference
-
1. Gormley EA, Lightner DJ, Faraday M, Vasavada SP. American Urological Association. Society of Urodynamics, Female Pelvic Medicine. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: AUA/SUFU guideline amendment. J Urol. 2015; 193:1572–1580. DOI: 10.1016/j.juro.2015.01.087. PMID: 25623739.
Article2. Danforth DN. Danforth's obstetrics and gynecology. 10 ed. Lippincott Williams & Wilkins;2008. p. 890–891.3. Ganz ML, Smalarz AM, Krupski TL, Anger JT, Hu JC, Wittrup-Jensen KU, et al. Economic costs of overactive bladder in the United States. Urology. 2010; 75:526–532. 532.e1–532.e18. DOI: 10.1016/j.urology.2009.06.096. PMID: 20035977.
Article4. Nazarudheen S, Dey S, Kandhwal K, Arora R, Reyar S, Khuroo AH, et al. Combining benefits of an adrenergic and a muscarinic blocker in a single formulation–A pharmacokinetic evaluation. Regul Toxicol Pharmacol. 2013; 67:226–231. DOI: 10.1016/j.yrtph.2013.07.015. PMID: 23933032.5. Jasek W, editor. Austria-Codex. 62nd ed. Vienna: Österreichischer Apothekerverlag;2007. p. 8659–8662. (in German).6. Astellas Pharma US I. VESIcare® (solifenacin succinate) tablet Label. 2010. Accessed 9 May 2018. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021518s008lbl.pdf.7. Uchida T, Krauwinkel WJ, Mulder H, Smulders RA. Food does not affect the pharmacokinetics of solifenacin, a new muscarinic receptor antagonist: results of a randomized crossover trial. Br J Clin Pharmacol. 2004; 58:4–7. PMID: 15206986.
Article8. Doroshyenko O, Fuhr U. Clinical pharmacokinetics and pharmacodynamics of solifenacin. Clin Pharmacokinet. 2009; 48:281–302. DOI: 10.2165/00003088-200948050-00001. PMID: 19566112.
Article9. Hanmi Pharmaceuticals Inc. S, Republic of Korea. Besigum® Tab. 2017. Accessed 9 May 2018. http://www.hanmi.co.kr/upfile/ces/product/e86a65fe-f08c-4438-86db-fbd4342724a2.pdf.10. MFDS. Recommendations for bioequivalence studies, Solifenacin succinate. 2013. Accessed 9 May 2018. http://drug.mfds.go.kr/html/boardLinkBody.jsp?p_menuId=0201&p_boardSeq=15&p_seq=1990&p_sub_menuId=020105.11. MFDS. Standard on Pharmaceutical Equivalence Study No. 2017-28. 2017. 14–15. Accessed 9 May 2018. http://mfds.go.kr/index.do?mid=687.12. FDA. Draft Guidance on Solifenacin Succinate. 2008. Accessed 9 May 2018. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm090334.pdf.13. FDA. Bioequivalence Studies with Pharmacokinetic Endpoints for Drugs Submitted Under an ANDA. 2013. Accessed 9 May 2018. https://www.fda.gov/downloads/drugs/guidances/ucm377465.pdf.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy of Solifenacin on Irritable Bowel Syndrome With Diarrhea: Open-label Prospective Pilot Trial
- Are Solifenacin and Ramosetron Really Ideal to Treat Irritable Bowel Syndrome?: Author's Reply
- Influence of Daytime or Nighttime Dosing with Solifenacin for Overactive Bladder with Nocturia: Impact on Nocturia and Sleep Quality
- Are Solifenacin and Ramosetron Really Ideal to Treat Irritable Bowel Syndrome?
- Meta-Analysis of the Efficacy and Safety of Mirabegron Add-On Therapy to Solifenacin for Overactive Bladder