J Vet Sci.
2016 Sep;17(3):261-268. 10.4142/jvs.2016.17.3.261.
Transgenesis for pig models
- Affiliations
-
- 1Laboratory of Theriogenology and Biotechnology, Department of Veterinary Clinical Science, College of Veterinary Medicine and the Research Institute of Veterinary Science, Seoul National University, Seoul 08826, Korea. snujang@snu.ac.kr
- 2Department of Biotechnology & Laboratory Animals, Shingu College, Seongnam 13174, Korea.
- 3Department of Chemistry, College of Natural Sciences, Seoul National University, Seoul 08826, Korea.
- 4Emergence Center for Food-Medicine Personalized Therapy System, Advanced Institutes of Convergence Technology, Seoul National University, Suwon 16229, Korea.
- 5Farm Animal Clinical Training and Research Center, Institutes of GreenBio Science Technology, Seoul National University, Pyeongchang 25354, Korea.
- KMID: 2413124
- DOI: http://doi.org/10.4142/jvs.2016.17.3.261
Abstract
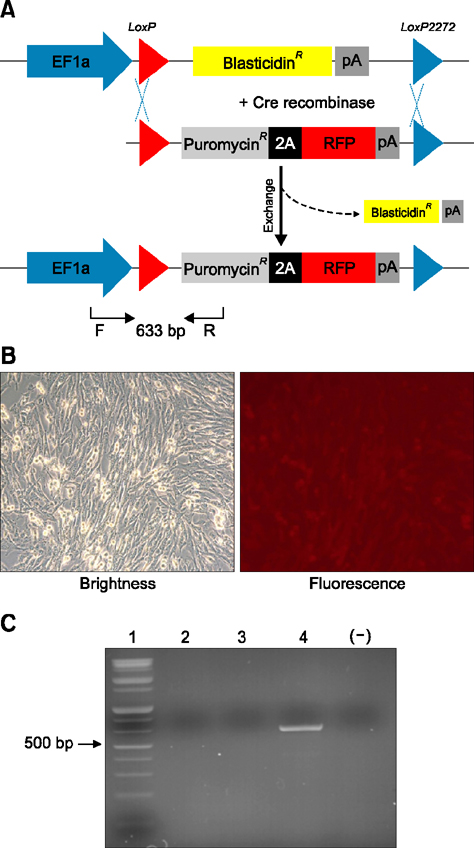
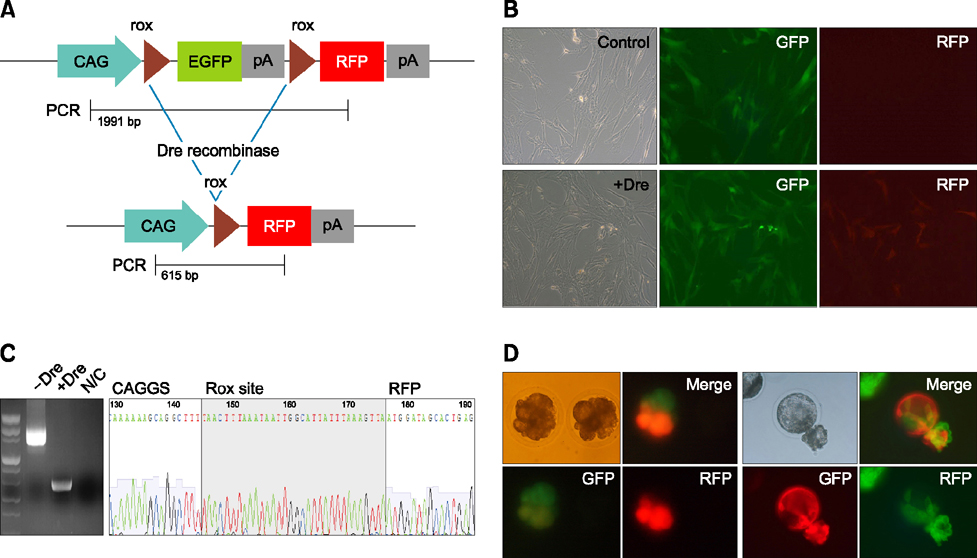
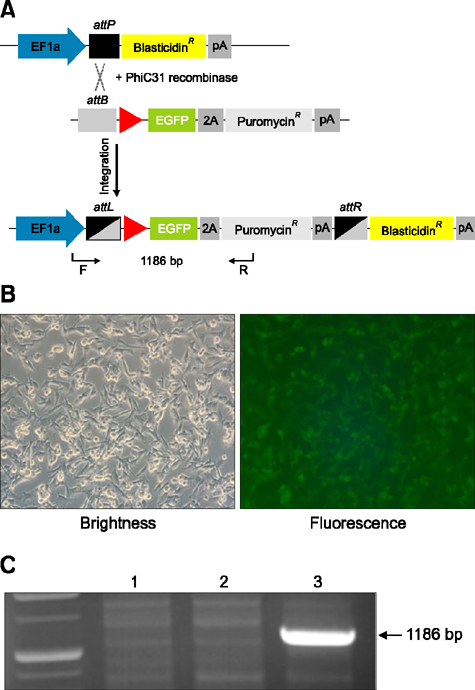
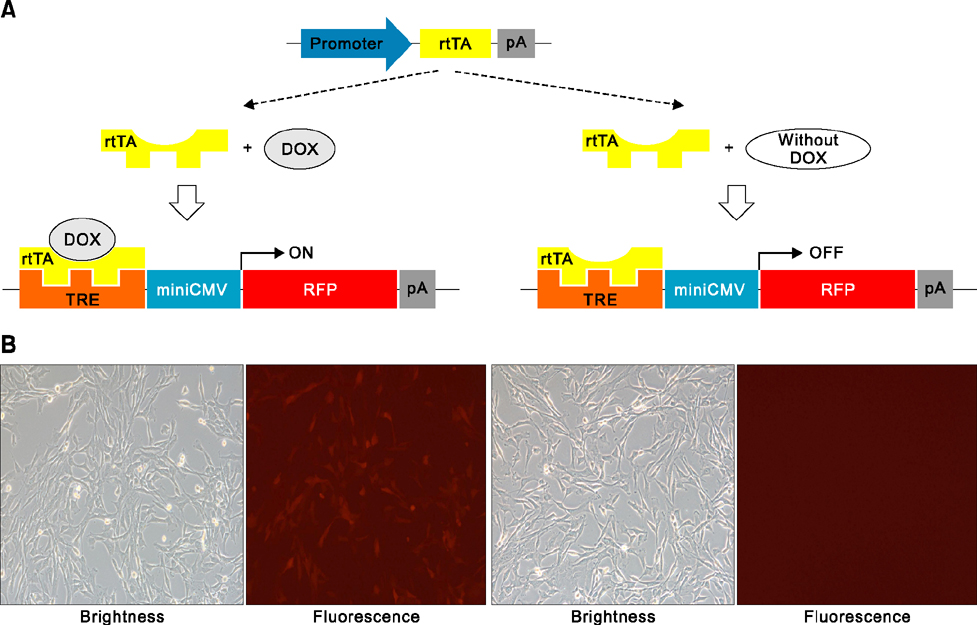
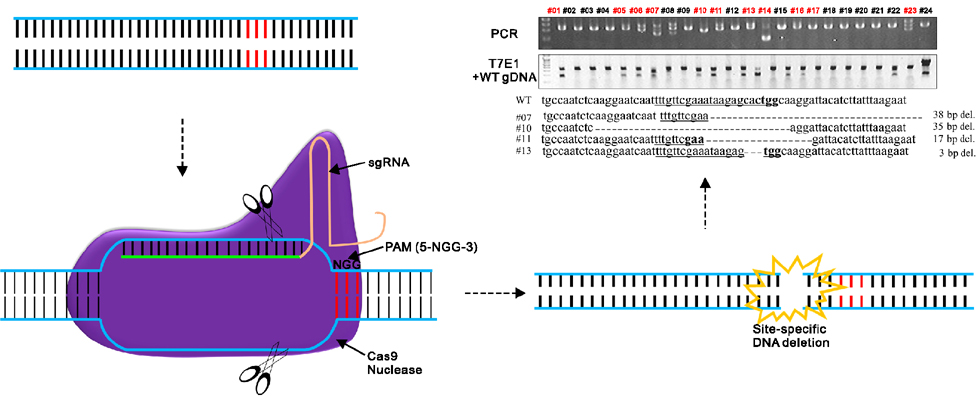
- Animal models, particularly pigs, have come to play an important role in translational biomedical research. There have been many pig models with genetically modifications via somatic cell nuclear transfer (SCNT). However, because most transgenic pigs have been produced by random integration to date, the necessity for more exact gene-mutated models using recombinase based conditional gene expression like mice has been raised. Currently, advanced genome-editing technologies enable us to generate specific gene-deleted and -inserted pig models. In the future, the development of pig models with gene editing technologies could be a valuable resource for biomedical research.
Keyword
MeSH Terms
Figure
Reference
-
1. Anastassiadis K, Fu J, Patsch C, Hu S, Weidlich S, Duerschke K, Buchholz F, Edenhofer F, Stewart AF. Dre recombinase, like Cre, is a highly efficient site-specific recombinase in E. coli, mammalian cells and mice. Dis Model Mech. 2009; 2:508–515.
Article2. Bi Y, Liu X, Zhang L, Shao C, Ma Z, Hua Z, Zhang L, Li L, Hua W, Xiao H, Wei Q, Zheng X. Pseudo attP sites in favor of transgene integration and expression in cultured porcine cells identified by Streptomyces phage phiC31 integrase. BMC Mol Biol. 2013; 14:20.
Article3. Branda CS, Dymecki SM. Talking about a revolution: the impact of site-specific recombinases on genetic analyses in mice. Dev Cell. 2004; 6:7–28.4. Brault V, Besson V, Magnol L, Duchon A, Hérault Y. Cre/loxP-mediated chromosome engineering of the mouse genome. Handb Exp Pharmacol. 2007; 29–48.5. Chan AWS, Kukolj G, Skalka AM, Bremel RD. Timing of DNA integration, transgenic mosaicism, and pronuclear microinjection. Mol Reprod Dev. 1999; 52:406–413.
Article6. Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981; 292:154–156.
Article7. Flisikowska T, Kind A, Schnieke A. The new pig on the block: modelling cancer in pigs. Transgenic Res. 2013; 22:673–680.
Article8. Garrels W, Mátés L, Holler S, Dalda A, Taylor U, Petersen B, Niemann H, Izsvák Z, Ivics Z, Kues WA. Germline transgenic pigs by Sleeping Beauty transposition in porcine zygotes and targeted integration in the pig genome. PLoS One. 2011; 6:e23573.
Article9. Grupen CG. The evolution of porcine embryo in vitro production. Theriogenology. 2014; 81:24–37.10. Gun G, Kues WA. Current progress of genetically engineered pig models for biomedical research. Biores Open Access. 2014; 3:255–264.
Article11. Hammer RE, Pursel VG, Rexroad CE Jr, Wall RJ, Bolt DJ, Ebert KM, Palmiter RD, Brinster RL. Production of transgenic rabbits, sheep and pigs by microinjection. Nature. 1985; 315:680–683.
Article12. Hauschild J, Petersen B, Santiago Y, Queisser AL, Carnwath JW, Lucas-Hahn A, Zhang L, Meng X, Gregory PD, Schwinzer R, Cost GJ, Niemann H. Efficient generation of a biallelic knockout in pigs using zinc-finger nucleases. Proc Natl Acad Sci U S A. 2011; 108:12013–12017.
Article13. Jin YX, Jeon Y, Lee SH, Kwon MS, Kim T, Cui XS, Hyun SH, Kim NH. Production of pigs expressing a transgene under the control of a tetracycline-inducible system. PLoS One. 2014; 9:e86146.
Article14. Klymiuk N, Böcker W, Schönitzer V, Bähr A, Radic T, Fröhlich T, Wünsch A, Keßler B, Kurome M, Schilling E, Herbach N, Wanke R, Nagashima H, Mutschler W, Arnold GJ, Schwinzer R, Schieker M, Wolf E. First inducible transgene expression in porcine large animal models. FASEB J. 2012; 26:1086–1099.
Article15. Kong Q, Hai T, Ma J, Huang T, Jiang D, Xie B, Wu M, Wang J, Song Y, Wang Y, He Y, Sun J, Hu K, Guo R, Wang L, Zhou Q, Mu Y, Liu Z. Rosa26 locus supports tissue-specific promoter driving transgene expression specifically in pig. PLoS One. 2014; 9:e107945.
Article16. Kragh PM, Nielsen AL, Li J, Du Y, Lin L, Schmidt M, Bøgh IB, Holm IE, Jakobsen MG, Purup S, Bolund L, Vajta G, Jørgensen AL. Hemizygous minipigs produced by random gene insertion and handmade cloning express the Alzheimer’s disease-causing dominant mutation APPsw. Transgenic Res. 2009; 18:545–558.
Article17. Kwon DN, Lee K, Kang MJ, Choi YJ, Park C, Whyte JJ, Brown AN, Kim JH, Samuel M, Mao J, Park KW, Murphy CN, Prather RS, Kim JH. Production of biallelic CMP-Neu5Ac hydroxylase knock-out pigs. Sci Rep. 2013; 3:1981.
Article18. Lai L, Kang JX, Li R, Wang J, Witt WT, Yong HY, Hao Y, Wax DM, Murphy CN, Rieke A, Samuel M, Linville ML, Korte SW, Evans RW, Starzl TE, Prather RS, Dai Y. Generation of cloned transgenic pigs rich in omega-3 fatty acids. Nat Biotechnol. 2006; 24:435–436.
Article19. Lai L, Park KW, Cheong HT, Kühholzer B, Samuel M, Bonk A, Im GS, Rieke A, Day BN, Murphy CN, Carter DN, Prather RS. Transgenic pig expressing the enhanced green fluorescent protein produced by nuclear transfer using colchicine-treated fibroblasts as donor cells. Mol Reprod Dev. 2002; 62:300–306.
Article20. Li L, Pang D, Wang T, Li Z, Chen L, Zhang M, Song N, Nie D, Chen Z, Lai L, Ouyang H. Production of a reporter transgenic pig for monitoring Cre recombinase activity. Biochem Biophys Res Commun. 2009; 382:232–235.
Article21. Luo W, Li Z, Huang Y, Han Y, Yao C, Duan X, Ouyang H, Li L. Generation of AQP2-Cre transgenic mini-pigs specifically expressing Cre recombinase in kidney collecting duct cells. Transgenic Res. 2014; 23:365–375.
Article22. Moon J, Kim S, Park H, Kang J, Park S, Koo O, da Torre BR, Saadeldin IM, Lee B. Production of porcine cloned embryos derived from cells conditionally expressing an exogenous gene using Cre-loxP. Zygote. 2012; 20:423–425.
Article23. Nagy A. Cre recombinase: the universal reagent for genome tailoring. Genesis. 2000; 26:99–109.
Article24. Nowak-Imialek M, Kues WA, Petersen B, Lucas-Hahn A, Herrmann D, Haridoss S, Oropeza M, Lemme E, Schöler HR, Carnwath JW, Niemann H. Oct4-enhanced green fluorescent protein transgenic pigs: a new large animal model for reprogramming studies. Stem Cells Dev. 2011; 20:1563–1575.
Article25. Onishi A, Iwamoto M, Akita T, Mikawa S, Takeda K, Awata T, Hanada H, Perry AC. Pig cloning by microinjection of fetal fibroblast nuclei. Science. 2000; 289:1188–1190.
Article26. Park CG, Bottino R, Hawthorne WJ. Current status of islet xenotransplantation. Int J Surg. 2015; 23:261–266.
Article27. Park SJ, Park HJ, Koo OJ, Choi WJ, Moon JH, Kwon DK, Kang JT, Kim S, Choi JY, Jang G, Lee BC. Oxamflatin improves developmental competence of porcine somatic cell nuclear transfer embryos. Cell Reprogram. 2012; 14:398–406.
Article28. Petersen B, Niemann H. Molecular scissors and their application in genetically modified farm animals. Transgenic Res. 2015; 24:381–396.
Article29. Phelps CJ, Koike C, Vaught TD, Boone J, Wells KD, Chen SH, Ball S, Specht SM, Polejaeva IA, Monahan JA, Jobst PM, Sharma SB, Lamborn AE, Garst AS, Moore M, Demetris AJ, Rudert WA, Bottino R, Bertera S, Trucco M, Starzl TE, Dai Y, Ayares DL. Production of α1,3-galactosyltransferase-deficient pigs. Science. 2003; 299:411–414.
Article30. Sajgo S, Ghinia MG, Shi M, Liu P, Dong L, Parmhans N, Popescu O, Badea TC. Dre - Cre sequential recombination provides new tools for retinal ganglion cell labeling and manipulation in mice. PLoS One. 2014; 9:e91435.
Article31. Sieren JC, Meyerholz DK, Wang XJ, Davis BT, Newell JD Jr, Hammond E, Rohret JA, Rohret FA, Struzynski JT, Goeken JA, Naumann PW, Leidinger MR, Taghiyev A, Van Rheeden R, Hagen J, Darbro BW, Quelle DE, Rogers CS. Development and translational imaging of a TP53 porcine tumorigenesis model. J Clin Invest. 2014; 124:4052–4066.
Article32. Takahagi Y, Fujimura T, Miyagawa S, Nagashima H, Shigehisa T, Shirakura R, Murakami H. Production of α1,3-galactosyltransferase gene knockout pigs expressing both human decay-accelerating factor and N-acetylglucosaminyltransferase III. Mol Reprod Dev. 2005; 71:331–338.
Article33. Tan W, Carlson DF, Lancto CA, Garbe JR, Webster DA, Hackett PB, Fahrenkrug SC. Efficient nonmeiotic allele introgression in livestock using custom endonucleases. Proc Natl Acad Sci U S A. 2013; 110:16526–16531.
Article34. Uchida M, Shimatsu Y, Onoe K, Matsuyama N, Niki R, Ikeda JE, Imai H. Production of transgenic miniature pigs by pronuclear microinjection. Transgenic Res. 2001; 10:577–582.35. Umeyama K, Watanabe M, Saito H, Kurome M, Tohi S, Matsunari H, Miki K, Nagashima H. Dominant-negative mutant hepatocyte nuclear factor 1α induces diabetes in transgenic-cloned pigs. Transgenic Res. 2009; 18:697–706.
Article36. Watanabe M, Umeyama K, Matsunari H, Takayanagi S, Haruyama E, Nakano K, Fujiwara T, Ikezawa Y, Nakauchi H, Nagashima H. Knockout of exogenous EGFP gene in porcine somatic cells using zinc-finger nucleases. Biochem Biophys Res Commun. 2010; 402:14–18.
Article37. Webster NL, Forni M, Bacci ML, Giovannoni R, Razzini R, Fantinati P, Zannoni A, Fusetti L, Dalprà L, Bianco MR, Papa M, Seren E, Sandrin MS, Mc Kenzie IF, Lavitrano M. Multi-transgenic pigs expressing three fluorescent proteins produced with high efficiency by sperm mediated gene transfer. Mol Reprod Dev. 2005; 72:68–76.
Article38. Wei J, Ouyang H, Wang Y, Pang D, Cong NX, Wang T, Leng B, Li D, Li X, Wu R, Ding Y, Gao F, Deng Y, Liu B, Li Z, Lai L, Feng H, Liu G, Deng X. Characterization of a hypertriglyceridemic transgenic miniature pig model expressing human apolipoprotein CIII. FEBS J. 2012; 279:91–99.
Article39. Whitworth KM, Lee K, Benne JA, Beaton BP, Spate LD, Murphy SL, Samuel MS, Mao J, O'Gorman C, Walters EM, Murphy CN, Driver J, Mileham A, McLaren D, Wells KD, Prather RS. Use of the CRISPR/Cas9 system to produce genetically engineered pigs from in vitro-derived oocytes and embryos. Biol Reprod. 2014; 91:78.40. Yao J, Huang J, Hai T, Wang X, Qin G, Zhang H, Wu R, Cao C, Xi JJ, Yuan Z, Zhao J. Efficient bi-allelic gene knockout and site-specific knock-in mediated by TALENs in pigs. Sci Rep. 2014; 4:6926.
Article41. Yu Y, Tong Q, Li Z, Tian J, Wang Y, Su F, Wang Y, Liu J, Zhang Y. Improved site-specific recombinase-based method to produce selectable marker- and vector-backbone-free transgenic cells. Sci Rep. 2014; 4:4240.
Article42. Zhang P, Liu P, Dou H, Chen L, Chen L, Lin L, Tan P, Vajta G, Gao J, Du Y, Ma RZ. Handmade cloned transgenic sheep rich in omega-3 fatty acids. PLoS One. 2013; 8:e55941.
Article43. Zhao J, Ross JW, Hao Y, Spate LD, Walters EM, Samuel MS, Rieke A, Murphy CN, Prather RS. Significant improvement in cloning efficiency of an inbred miniature pig by histone deacetylase inhibitor treatment after somatic cell nuclear transfer. Biol Reprod. 2009; 81:525–530.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Blood Pressure Regulation by Vasoactive Peptide Genes: Transgenic And Knockout Animal Models
- Genetically Engineered Mouse Models for Drug Development and Preclinical Trials
- Research Progress and Future Perspectives in Animal Stem Cell Research
- Awareness on Zoonoses among Pig Farmers in Korea
- Study of the Experimental Dermatophyte Infection in Animals