Yonsei Med J.
2018 Jul;59(5):633-642. 10.3349/ymj.2018.59.5.633.
Effects of the Helicobacter pylori Virulence Factor CagA and Ammonium Ion on Mucins in AGS Cells
- Affiliations
-
- 1Department of Gastroenterology, Henan University of Chinese Medicine, Zhengzhou, China.
- 2Department of Gastroenterology, Ningbo No. 2 Hospital, Ningbo, China. shidingyuhang@163.com
- 3Department of Gastroenterology, the First People's Hospital of Yuhang District, Hangzhou, China.
- KMID: 2412654
- DOI: http://doi.org/10.3349/ymj.2018.59.5.633
Abstract
- PURPOSE
To investigate the effects of Helicobacter pylori (H. pylori)-CagA and the urease metabolite NH₄⺠on mucin expression in AGS cells.
MATERIALS AND METHODS
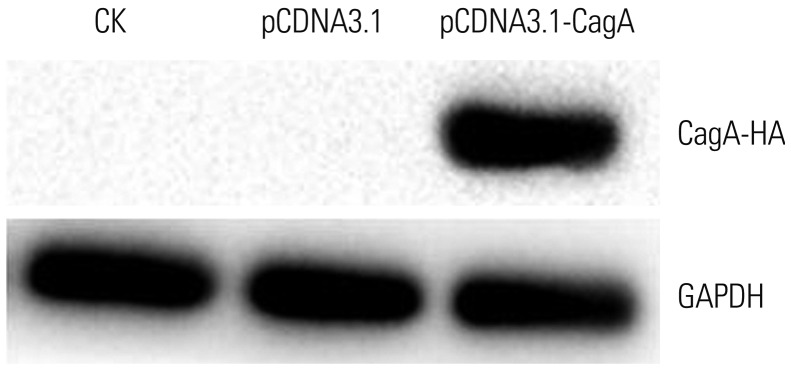
AGS cells were transfected with CagA and/or treated with different concentrations of NHâ‚„âºCL. Mucin gene and protein expression was assessed by qPCR and immunofluorescence assays, respectively.
RESULTS
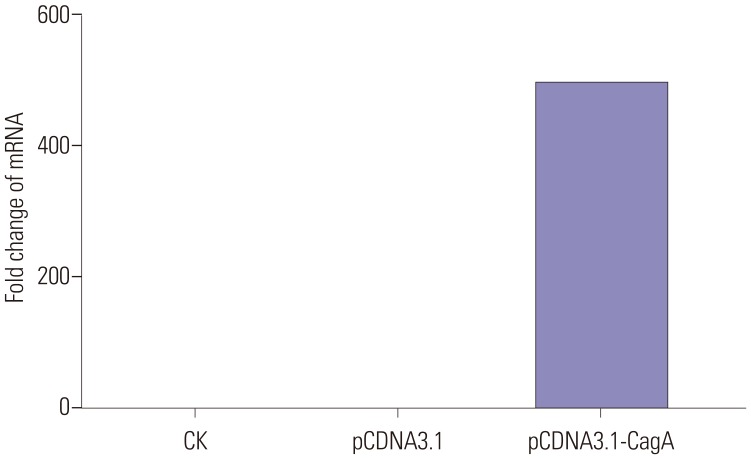
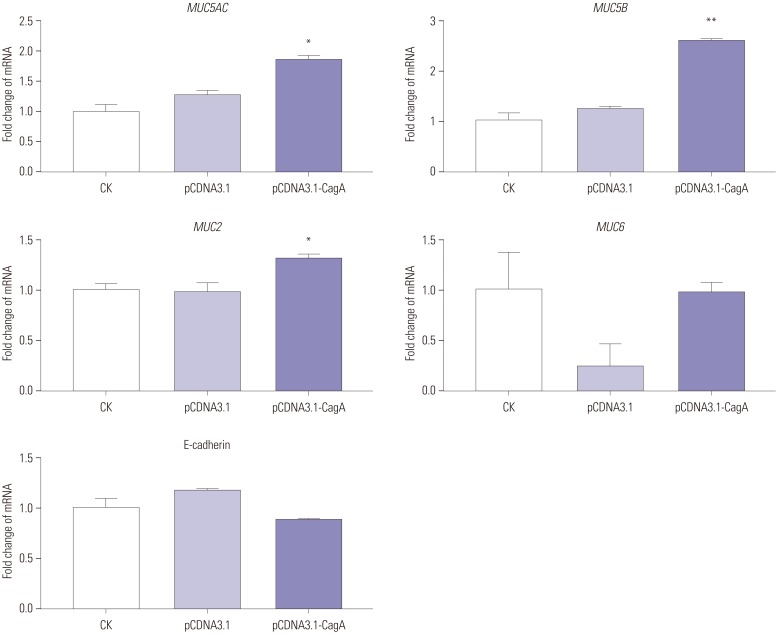
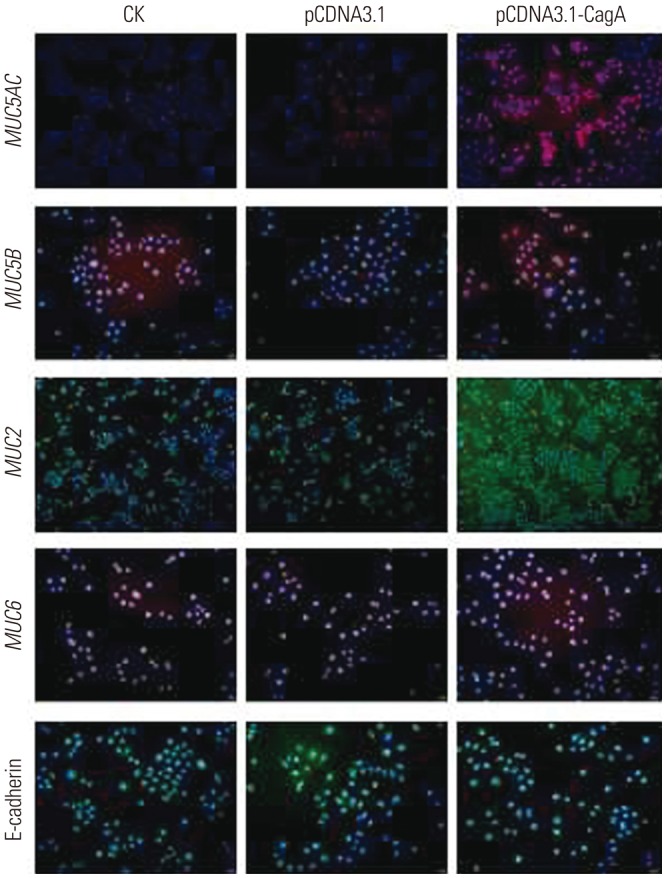
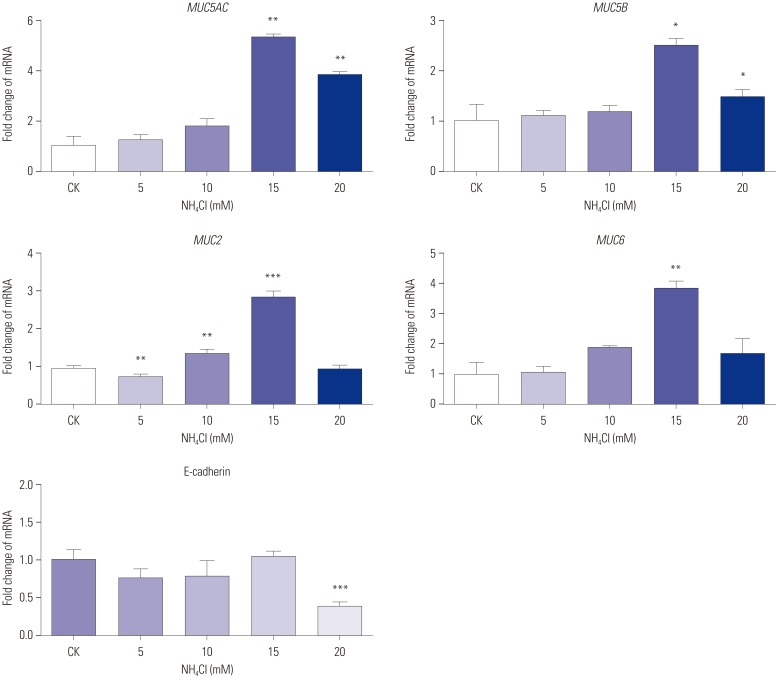
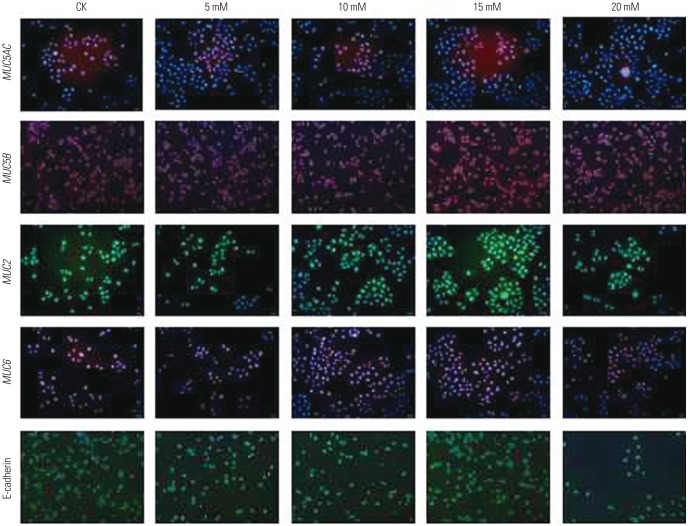
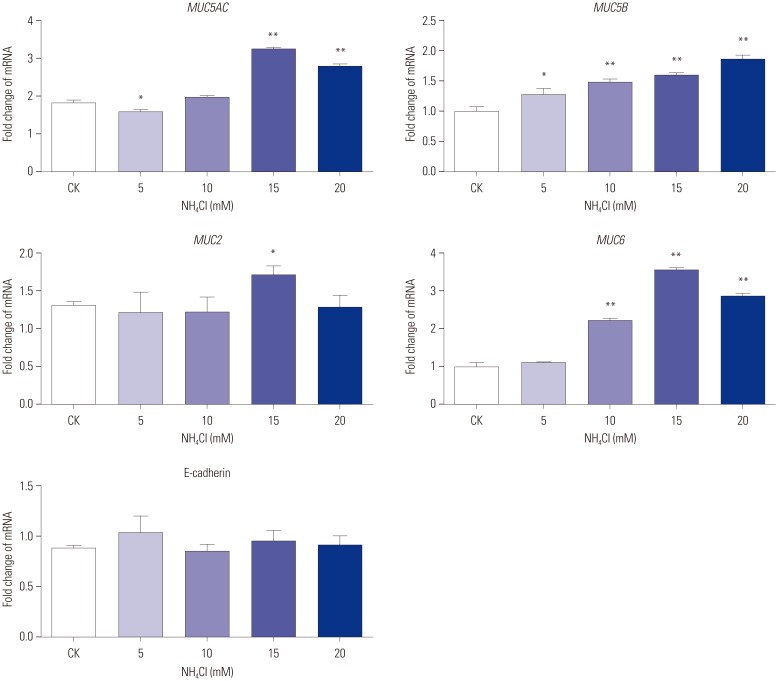
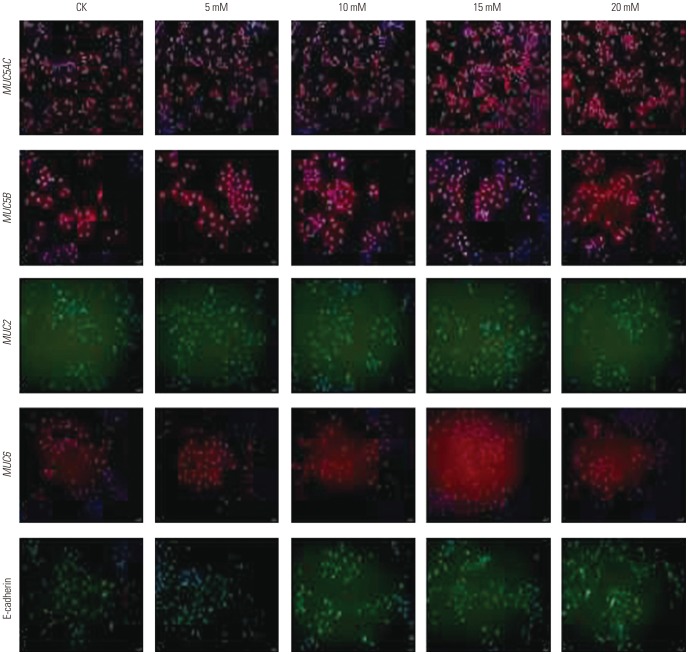
CagA significantly upregulated MUC5AC, MUC2, and MUC5B expression in AGS cells, but did not affect E-cadherin and MUC6 expression. MUC5AC, MUC6, and MUC2 expression in AGS cells increased with increasing NH₄⺠concentrations until reaching a peak level at 15 mM. MUC5B mRNA expression in AGS cells (NH₄⺠concentration of 15 mM) was significantly higher than that at 0, 5, and 10 mM NHâ‚„âº. No changes in E-cadherin expression in AGS cells treated with NH₄⺠were noted, except at 20 mM. The expression of MUC5AC, MUC2, and MUC6 mRNA in CagA-transfected AGS cells at an NH₄⺠concentration of 15 mM was significantly NH₄⺠concentration, and was significantly higher compared to that in untreated cells. No significant change in the expression of E-cadherin mRNA in CagA-transfected AGS cells was observed. Immunofluorescence assays confirmed the observed changes.
CONCLUSION
H. pylori may affect the expression of MUC5AC, MUC2, MUC5B, and MUC6 in AGS cells via CagA and/or NHâ‚„âº, but not E-cadherin.
Keyword
MeSH Terms
Figure
Reference
-
1. Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004; 4:45–60. PMID: 14681689.
Article2. Babu SD, Jayanthi V, Devaraj N, Reis CA, Devaraj H. Expression profile of mucins (MUC2, MUC5AC and MUC6) in Helicobacter pylori infected pre-neoplastic and neoplastic human gastric epithelium. Mol Cancer. 2006; 5:10. PMID: 16545139.
Article3. Shi D, Qiu XM, Bao YF. Effects of Helicobacter pylori infection on MUC5AC protein expression in gastric cancer. Future Oncol. 2013; 9:115–120. PMID: 23252568.
Article4. Gomceli I, Demiriz B, Tez M. Gastric carcinogenesis. World J Gastroenterol. 2012; 18:5164–5170. PMID: 23066309.5. Van de Bovenkamp JH, Mahdavi J, Korteland-Van Male AM, Büller HA, Einerhand AW, Borén T, et al. The MUC5AC glycoprotein is the primary receptor for Helicobacter pylori in the human stomach. Helicobacter. 2003; 8:521–532. PMID: 14535999.
Article6. Shi D, Qiu XM, Yan XJ. The changes in MUC5AC expression in gastric cancer before and after Helicobacter pylori eradication. Clin Res Hepatol Gastroenterol. 2014; 38:235–240. PMID: 23910060.
Article7. Niv Y. Helicobacter pylori and gastric mucin expression: a systematic review and meta-analysis. World J Gastroenterol. 2015; 21:9430–9436. PMID: 26309370.8. Kang HM, Kim N, Park YS, Hwang JH, Kim JW, Jeong SH, et al. Effects of Helicobacter pylori Infection on gastric mucin expression. J Clin Gastroenterol. 2008; 42:29–35. PMID: 18097286.
Article9. McLean MH, El-Omar EM. Genetics of gastric cancer. Nat Rev Gastroenterol Hepatol. 2014; 11:664–674. PMID: 25134511.
Article10. Zhang BG, Hu L, Zang MD, Wang HX, Zhao W, Li JF, et al. Helicobacter pylori CagA induces tumor suppressor gene hypermethylation by upregulating DNMT1 via AKT-NFκB pathway in gastric cancer development. Oncotarget. 2016; 7:9788–9800. PMID: 26848521.
Article11. Tohidpour A. CagA-mediated pathogenesis of Helicobacter pylori. Microb Pathog. 2016; 93:44–55. PMID: 26796299.
Article12. Figura N, Marano L, Moretti E, Ponzetto A. Helicobacter pylori infection and gastric carcinoma: not all the strains and patients are alike. World J Gastrointest Oncol. 2016; 8:40–54. PMID: 26798436.13. Perrais M, Rousseaux C, Ducourouble MP, Courcol R, Vincent P, Jonckheere N, et al. Helicobacter pylori urease and flagellin alter mucin gene expression in human gastric cancer cells. Gastric Cancer. 2014; 17:235–246. PMID: 23703470.
Article14. Wen R, Gao F, Zhou CJ, Jia YB. Polymorphisms in mucin genes in the development of gastric cancer. World J Gastrointest Oncol. 2015; 7:328–337. PMID: 26600932.
Article15. Pinto-de-Sousa J, Reis CA, David L, Pimenta A, Cardoso-de-Oliveira M. MUC5B expression in gastric carcinoma: relationship with clinico-pathological parameters and with expression of mucins MUC1, MUC2, MUC5AC and MUC6. Virchows Arch. 2004; 444:224–230. PMID: 14758553.
Article16. Van De Bovenkamp JH, Korteland-Van Male AM, Büller HA, Einerhand AW, Dekker J. Infection with Helicobacter pylori affects all major secretory cell populations in the human antrum. Dig Dis Sci. 2005; 50:1078–1086. PMID: 15986858.
Article17. Byrd JC, Yan P, Sternberg L, Yunker CK, Scheiman JM, Bresalier RS. Aberrant expression of gland-type gastric mucin in the surface epithelium of Helicobacter pylori-infected patients. Gastroenterology. 1997; 113:455–464. PMID: 9247464.
Article18. Zheng H, Takahashi H, Nakajima T, Murai Y, Cui Z, Nomoto K, et al. MUC6 down-regulation correlates with gastric carcinoma progression and a poor prognosis: an immunohistochemical study with tissue microarrays. J Cancer Res Clin Oncol. 2006; 132:817–823. PMID: 16807756.
Article19. Reis CA, David L, Carvalho F, Mandel U, de Bolós C, Mirgorodskaya E, et al. Immunohistochemical study of the expression of MUC6 mucin and co-expression of other secreted mucins (MUC5AC and MUC2) in human gastric carcinomas. J Histochem Cytochem. 2000; 48:377–388. PMID: 10681391.
Article20. Ho SB, Shekels LL, Toribara NW, Kim YS, Lyftogt C, Cherwitz DL, et al. Mucin gene expression in normal, preneoplastic, and neoplastic human gastric epithelium. Cancer Res. 1995; 55:2681–2690. PMID: 7780985.21. Mejóas-Luque R, Lindén SK, Garrido M, Tye H, Najdovska M, Jenkins BJ, et al. Inflammation modulates the expression of the intestinal mucins MUC2 and MUC4 in gastric tumors. Oncogene. 2010; 29:1753–1762. PMID: 20062084.
Article22. Chan AO, Lam SK, Wong BC, Wong WM, Yuen MF, Yeung YH, et al. Promoter methylation of E-cadherin gene in gastric mucosa associated with Helicobacter pylori infection and in gastric cancer. Gut. 2003; 52:502–506. PMID: 12631658.
Article23. Meng W, Bai B, Sheng L, Li Y, Yue P, Li X, et al. Role of Helicobacter pylori in gastric cancer: advances and controversies. Discov Med. 2015; 20:285–293. PMID: 26645900.24. Chung WC, Jung SH, Joo KR, Kim MJ, Youn GJ, Kim Y, et al. An inverse relationship between the expression of the gastric tumor suppressor RUNX3 and infection with Helicobacter pylori in gastric epithelial dysplasia. Gut Liver. 2013; 7:688–695. PMID: 24312710.25. Matsuda K, Yamauchi K, Matsumoto T, Sano K, Yamaoka Y, Ota H. Quantitative analysis of the effect of Helicobacter pylori on the expressions of SOX2, CDX2, MUC2, MUC5AC, MUC6, TFF1, TFF2, and TFF3 mRNAs in human gastric carcinoma cells. Scand J Gastroenterol. 2008; 43:25–33. PMID: 18938748.26. Byrd JC, Yunker CK, Xu QS, Sternberg LR, Bresalier RS. Inhibition of gastric mucin synthesis by Helicobacter pylori. Gastroenterology. 2000; 118:1072–1079. PMID: 10833482.
Article27. Kocer B, Ulas M, Ustundag Y, Erdogan S, Karabeyoglu M, Yldrm O, et al. A confirmatory report for the close interaction of Helicobacter pylori with gastric epithelial MUC5AC expression. J Clin Gastroenterol. 2004; 38:496–502. PMID: 15220684.
Article28. Hayashi T, Senda M, Morohashi H, Higashi H, Horio M, Kashiba Y, et al. Tertiary structure-function analysis reveals the pathogenic signaling potentiation mechanism of Helicobacter pylori oncogenic effector CagA. Cell Host Microbe. 2012; 12:20–33. PMID: 22817985.
Article29. Wu J, Xu S, Zhu Y. Helicobacter pylori CagA: a critical destroyer of the gastric epithelial barrier. Dig Dis Sci. 2013; 58:1830–1837. PMID: 23423500.
Article30. Schmitz JM, Durham CG, Ho SB, Lorenz RG. Gastric mucus alterations associated with murine Helicobacter infection. J Histochem Cytochem. 2009; 57:457–467. PMID: 19153195.31. Reis CA, David L, Correa P, Carneiro F, de Bolós C, Garcia E, et al. Intestinal metaplasia of human stomach displays distinct patterns of mucin (MUC1, MUC2, MUC5AC, and MUC6) expression. Cancer Res. 1999; 59:1003–1007. PMID: 10070955.32. Li N, Xu X, Xiao B, Zhu ED, Li BS, Liu Z, et al. H. pylori related proinflammatory cytokines contribute to the induction of miR-146a in human gastric epithelial cells. Mol Biol Rep. 2012; 39:4655–4661. PMID: 21947847.
Article33. Yu H, Zeng J, Liang X, Wang W, Zhou Y, Sun Y, et al. Helicobacter pylori promotes epithelial-mesenchymal transition in gastric cancer by downregulating programmed cell death protein 4 (PDCD4). PLoS One. 2014; 9:e105306. PMID: 25144746.
Article34. Wagih HM, El-Ageery SM, Alghaithy AA. A study of RUNX3, Ecadherin and β-catenin in CagA-positive Helicobacter pylori associated chronic gastritis in Saudi patients. Eur Rev Med Pharmacol Sci. 2015; 19:1416–1429. PMID: 25967717.35. Yu XW, Xu Q, Xu Y, Gong YH, Yuan Y. Expression of the E-cadherin/ β-catenin/tcf-4 pathway in gastric diseases with relation to Helicobacter pylori infection: clinical and pathological implications. Asian Pac J Cancer Prev. 2014; 15:215–220. PMID: 24528029.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum to “Effects of the Helicobacter pylori Virulence Factor CagA and Ammonium Ion on Mucins in AGS Cells” by Zhang X, et al.
- Perspective of Helicobacter pylori Research: Molecular Pathogenesis of Helicobacter pylori Virulence Factors
- Prevalence of Virulence Factors in Helicobacter pylori Isolated from Koreans and Proinflammatory Cytokine Gene Expression in Human Gastric Epithelial Cells Induced by H. pylori with Virulence Factors
- Proinflammatory Cytokine Gene Expression in Human Gastric Epithelial Cells Induced by H. pylori According to Virulence Factors
- The Positive Rates of Helicobacter pylori cagA Gene in Gastric Biopsy Specimens of the Patients with Gastritis , Gastric Ulcer , Duodenal Ulcer , and Gastric Cancer and Comparison of the Degree of Gastritis