Allergy Asthma Respir Dis.
2018 May;6(3):155-160. 10.4168/aard.2018.6.3.155.
Clinical features of Mycoplasma pneumonia in comparison with viral pneumoina in children: A multicenter, cross-sectional study
- Affiliations
-
- 1Department of Pediatrics, Kangdong Sacred Heart Hospital, Seoul, Korea. paviola7@gmail.com
- 2Department of Pediatrics, Inje University Haeundae Paik Hospital, Busan, Korea.
- 3Department of Pediatrics, Myongji Hospital, Seonam University College of Medicine, Goyang, Korea.
- 4Department of Pediatrics, Kyung Hee University Medical Center, Kyung Hee University School of Medicine, Seoul, Korea.
- 5Department of Pediatrics, CHA Gangnam Medical Center, CHA University School of Medicine, Seoul, Korea.
- 6Department of Medicine, CHA Bundang Medical Center, CHA University School of Medicine, Seongnam, Korea.
- KMID: 2412518
- DOI: http://doi.org/10.4168/aard.2018.6.3.155
Abstract
- PURPOSE
This study was conducted to compare clinical features between Mycoplasma pneumonia and viral pneumonia.
METHODS
We retrospectively analyzed the medical records of 428 patients requiring hospitalization among children younger than 18 years of age in 5 hospitals in Seoul and Gyeonggi-do. There were 131 patients with M. pneumonia and virus coinfection, 167 patients with M. pneumonia without virus coinfection, and 130 patients with viral pneumonia. All subjects had radiographic evidence of pneumonia with specimens available for both M. pneumonia and viral testing. Virus was identified using the polymerase chain reaction assay in a nasopharyngeal or oropharyngeal swab. M. pneumoniae pneumonia was diagnosed serologically.
RESULTS
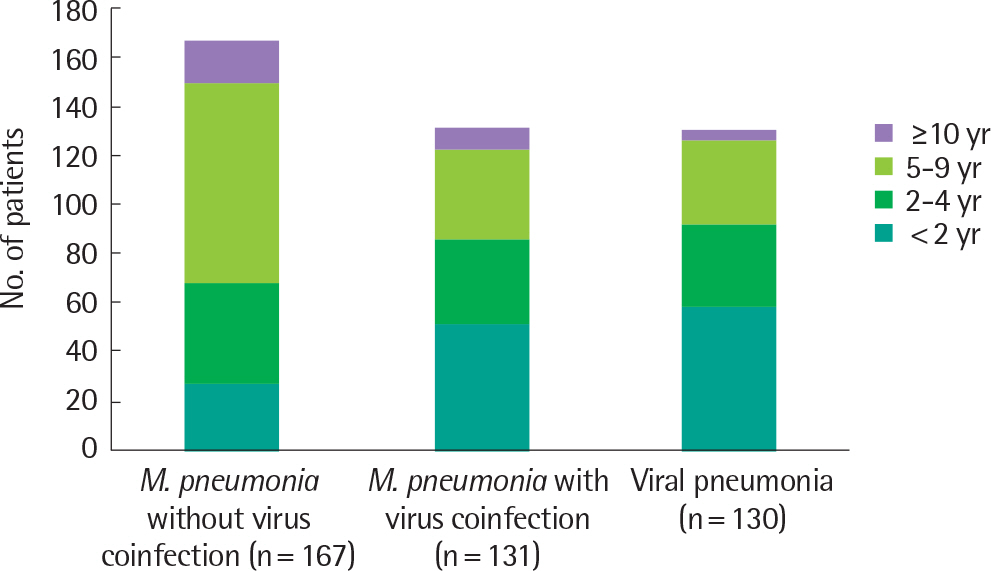
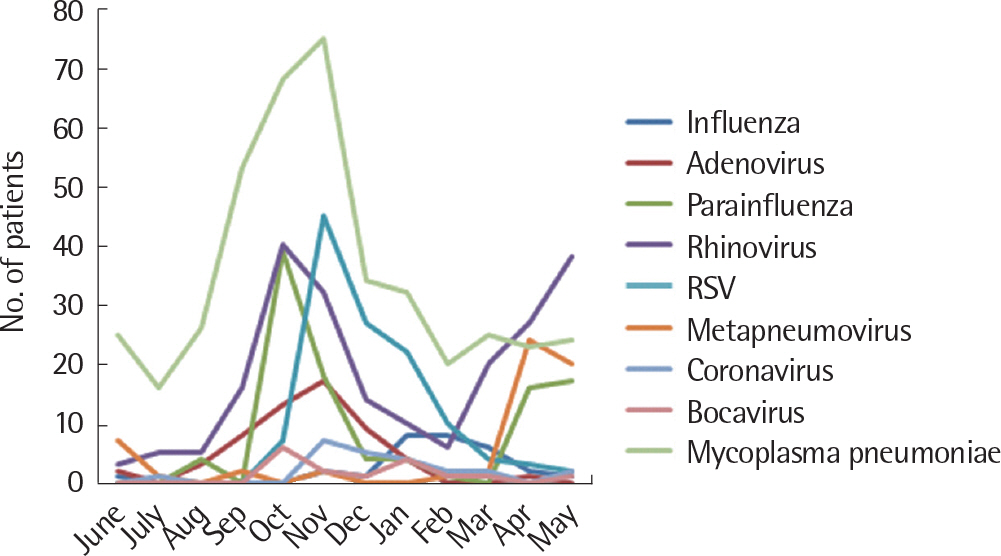
Human rhinovirus was detected in 60.3% (79 of 131) of children with M. pneumonia accompanied by virus coinfection. Respiratory syncytial virus (RSV) was detected in 38.2% (50 of 130) of children with viral pneumonia. The mean age was significantly lower in the viral pneumonia group than in the M. pneumonia group with and without virus coinfection. The sex distribution did not differ significantly among the 3 study groups. The procalcitonin level was higher in viral pneumonia and erythrocyte sedimentation rate level was higher in the M. pneumonia group although no significant difference was found in C-reactive protein level between the M. pneumonia and viral pneumonia groups.
CONCLUSION
Clinical features and inflammatory markers between M. pneumonia and viral pneumonia may be useful for the treatment of community-acquired pneumonia.
MeSH Terms
-
Blood Sedimentation
C-Reactive Protein
Child*
Coinfection
Cross-Sectional Studies*
Gyeonggi-do
Hospitalization
Humans
Medical Records
Mycoplasma*
Pneumonia
Pneumonia, Mycoplasma*
Pneumonia, Viral
Polymerase Chain Reaction
Respiratory Syncytial Viruses
Retrospective Studies
Rhinovirus
Seoul
Sex Distribution
C-Reactive Protein
Figure
Reference
-
1. Pfuntner A, Wier LM, Stocks C. Most frequent conditions in U.S. Hospitals, 2011: Statistical Brief #162. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): Agency for Healthcare Research and Quality (US);2006.2. Lee GE, Lorch SA, Sheffler-Collins S, Kronman MP, Shah SS. National hospitalization trends for pediatric pneumonia and associated complications. Pediatrics. 2010; 126:204–13.
Article3. Eun BW, Kim NH, Choi EH, Lee HJ. Mycoplasma pneumoniae in Korean children: the epidemiology of pneumonia over an 18-year period. J Infect. 2008; 56:326–31.
Article4. Niitu Y, Hasegawa S, Suetake T, Kubota H, Komatsu S, Horikawa M. Resistance of Mycoplasma pneumoniae to erythromycin and other antibiotics. J Pediatr. 1970; 76:438–43.
Article5. Dumke R, von Baum H, Luck PC, Jacobs E. Occurrence of macrolide-re-sistant Mycoplasma pneumoniae strains in Germany. Clin Microbiol Infect. 2010; 16:613–6.
Article6. Peuchant O, Ménard A, Renaudin H, Morozumi M, Ubukata K, Bébéar CM, et al. Increased macrolide resistance of Mycoplasma pneumoniae in France directly detected in clinical specimens by realtime PCR and melting curve analysis. J Antimicrob Chemother. 2009; 64:52–8.
Article7. Okada T, Morozumi M, Tajima T, Hasegawa M, Sakata H, Ohnari S, et al. Rapid effectiveness of minocycline or doxycycline against macrolide-re-sistant Mycoplasma pneumoniae infection in a 2011 outbreak among Japanese children. Clin Infect Dis. 2012; 55:1642–9.
Article8. Chang MW, Kim KH, Park ID, Song GY, Kim SW, Lee EY, et al. Isolation of Mycoplasma pneumoniae and antimicrobial susceptibilities of the isolates(III). J Life Sci. 2005; 15:479–85.
Article9. Hong KB, Choi EH, Lee HJ, Lee SY, Cho EY, Choi JH, et al. Macrolide resistance of Mycoplasma pneumoniae, South Korea, 2000-2011. Emerg Infect Dis. 2013; 19:1281–4.10. Lee E, Cho HJ, Hong SJ, Lee J, Sung H, Yu J. Prevalence and clinical manifestations of macrolide resistant Mycoplasma pneumoniae pneumonia in Korean children. Korean J Pediatr. 2017; 60:151–7.
Article11. Ahn HS, Shin HY, editors. Hong Chang Eui pediatrics. 11th ed.Seoul: Mirae N;2016. p. 697.12. Sensini A, Zuccherini F, Cerboni1 G, Galullo M, Meli L, Maso GD, et al. Serological diagnosis of Mycoplasma pneumoniae infection: a complicated puzzle. Microbiol Med. 2012; 27:171–6.
Article13. Iwane MK, Prill MM, Lu X, Miller EK, Edwards KM, Hall CB, et al. Human rhinovirus species associated with hospitalizations for acute respiratory illness in young US children. J Infect Dis. 2011; 204:1702–10.
Article14. Hayden FG. Rhinovirus and the lower respiratory tract. Rev Med Virol. 2004; 14:17–31.
Article15. Jartti T, Lee WM, Pappas T, Evans M, Lemanske RF Jr, Gern JE. Serial viral infections in infants with recurrent respiratory illnesses. Eur Respir J. 2008; 32:314–20.
Article16. García-García ML, Calvo C, Pozo F, Villadangos PA, Pérez-Breña P, Casas I. Spectrum of respiratory viruses in children with community-acquired pneumonia. Pediatr Infect Dis J. 2012; 31:808–13.
Article17. Jain S, Williams DJ, Arnold SR, Ampofo K, Bramley AM, Reed C, et al. Community-acquired pneumonia requiring hospitalization among U.S. children. N Engl J Med. 2015; 372:835–45.
Article18. Pavia AT. Viral infections of the lower respiratory tract: old viruses, new viruses, and the role of diagnosis. Clin Infect Dis. 2011; 52(Suppl 4):S284–9.
Article19. Bradley JS, Byington CL, Shah SS, Alverson B, Carter ER, Harrison C, et al. The management of community-acquired pneumonia in infants and children older than 3 months of age: clinical practice guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin Infect Dis. 2011; 53:e25–76.
Article20. Jacobs JW, Lund PK, Potts JT Jr, Bell NH, Habener JF. Procalcitonin is a glycoprotein. J Biol Chem. 1981; 256:2803–7.
Article21. Reinhart K, Karzai W, Meisner M. Procalcitonin as a marker of the systemic inflammatory response to infection. Intensive Care Med. 2000; 26:1193–200.
Article22. Becker KL, Nylén ES, White JC, Müller B, Snider RH Jr. Clinical review 167: Procalcitonin and the calcitonin gene family of peptides in inflammation, infection, and sepsis: a journey from calcitonin back to its precursors. J Clin Endocrinol Metab. 2004; 89:1512–25.23. Müller B, Becker KL, Schächinger H, Rickenbacher PR, Huber PR, Zim-merli W, et al. Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Crit Care Med. 2000; 28:977–83.
Article24. Baer G, Baumann P, Buettcher M, Heininger U, Berthet G, Schäfer J, et al. Procalcitonin guidance to reduce antibiotic treatment of lower respiratory tract infection in children and adolescents (ProPAED): a randomized controlled trial. PLoS One. 2013; 8:e68419.
Article25. Esposito S, Tagliabue C, Picciolli I, Semino M, Sabatini C, Consolo S, et al. Procalcitonin measurements for guiding antibiotictreatment in pediatric pneumonia. Respir Med. 2011; 105:1939–45.26. Moulin F, Raymond J, Lorrot M, Marc E, Coste J, Iniguez JL, et al. Procalcitonin in children admitted to hospital with community acquired pneumonia. Arch Dis Child. 2001; 84:332–6.
Article27. Toikka P, Irjala K, Juvén T, Virkki R, Mertsola J, Leinonen M, et al. Serum procalcitonin, C-reactive protein and interleukin-6 for distinguishing bacterial and viral pneumonia in children. Pediatr Infect Dis J. 2000; 19:598–602.
Article28. Stockmann C, Ampofo K, Killpack J, Williams DJ, Edwards KM, Grijalva CG, et al. Procalcitonin accurately identifies hospitalized children with low risk of bacterial community-acquired pneumonia. J Pediatric Infect Dis Soc. 2018; 7:46–53.
Article29. Menéndez R, Sahuquillo-Arce JM, Reyes S, Martínez R, Polverino E, Cil-lóniz C, et al. Cytokine activation patterns and biomarkers are influenced by microorganisms in community-acquired pneumonia. Chest. 2012; 141:1537–45.
Article30. Nascimento-Carvalho CM, Cardoso MR, Barral A, Araújo-Neto CA, Guerin S, Saukkoriipi A, et al. Procalcitonin is useful in identifying bac-teraemia among children with pneumonia. Scand J Infect Dis. 2010; 42:644–9.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A clinical study of mycoplasma pneumonia in children during recent 5 years
- Clinical usefulness of serum procalcitonin to distinguish between viral pneumonia and Mycoplasma pneumonia in children: A multicenter, cross-sectional study
- Clinical Observation on Pneumonia due to Mycoplasma Pneumoniae in Children
- Clinical Study of Patients with Mycoplasma Pneumoniae Pneumonia in Children
- Mycoplasma pneumoniae Pneumonia in Children