Clin Orthop Surg.
2017 Dec;9(4):439-457. 10.4055/cios.2017.9.4.439.
A Randomized, Multicenter, Phase III Trial to Evaluate the Efficacy and Safety of Polmacoxib Compared with Celecoxib and Placebo for Patients with Osteoarthritis
- Affiliations
-
- 1Department of Orthopedic Surgery, Seoul National University Hospital, Seoul, Korea.
- 2Department of Orthopedic Surgery, National Health Insurance Service Ilsan Hospital, Goyang, Korea.
- 3Department of Orthopedic Surgery, Konkuk University Medical Center, Seoul, Korea.
- 4Clinical Research Center, Kyungpook National University Hospital, Daegu, Korea.
- 5Department of Orthopedic Surgery, Asan Medical Center, Seoul, Korea.
- 6Department of Orthopedic Surgery, SMG-SNU Boramae Medical Center, Seoul, Korea.
- 7Department of Orthopedic Surgery, Hanyang University Seoul Hospital, Seoul, Korea.
- 8Department of Orthopedic Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 9Clinical Research Center, Chungnam National University Hospital, Daejeon, Korea.
- 10Department of Orthopedic Surgery, Korea University Anam Hospital, Seoul, Korea.
- 11Department of Orthopedic Surgery, Seoul St. Mary's Hospital, Seoul, Korea.
- 12Department of Orthopedic Surgery, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 13Department of Orthopedic Surgery, Ewha Womans University Mokdong Hospital, Seoul, Korea.
- 14Department of Orthopedic Surgery, Korean Armed Forces Capital Hospital, Seongnam, Korea.
- 15Clinical Research Department, CG Pharmaceuticals, Inc., Orinda, CA, USA. scho@cgxinc.com
- KMID: 2412245
- DOI: http://doi.org/10.4055/cios.2017.9.4.439
Abstract
- BACKGROUND
The aim of this study was to evaluate the safety and analgesic efficacy of polmacoxib 2 mg versus placebo in a superiority comparison or versus celecoxib 200 mg in a noninferiority comparison in patients with osteoarthritis (OA).
METHODS
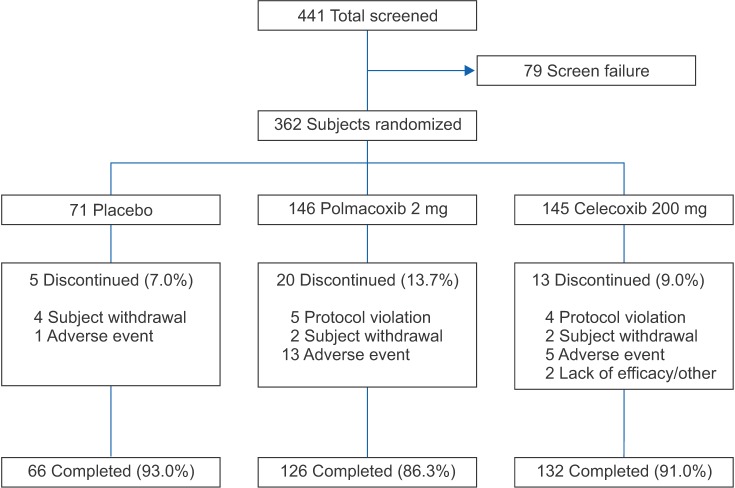
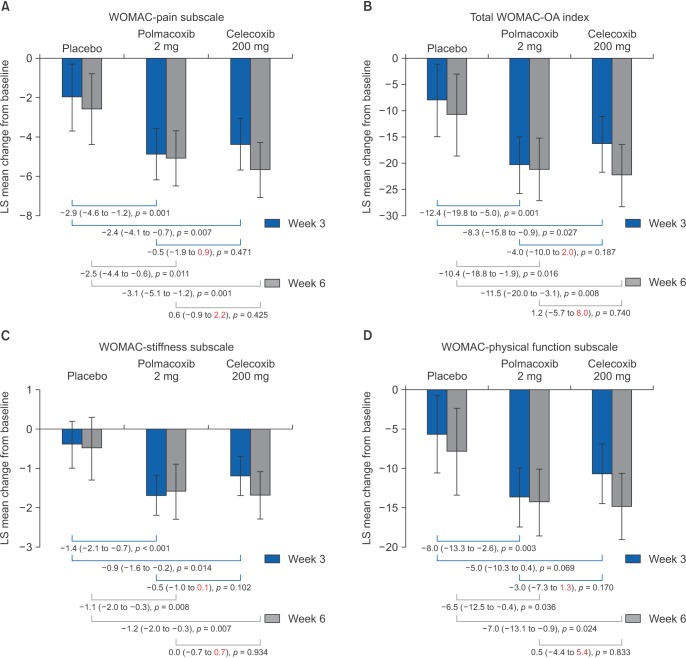
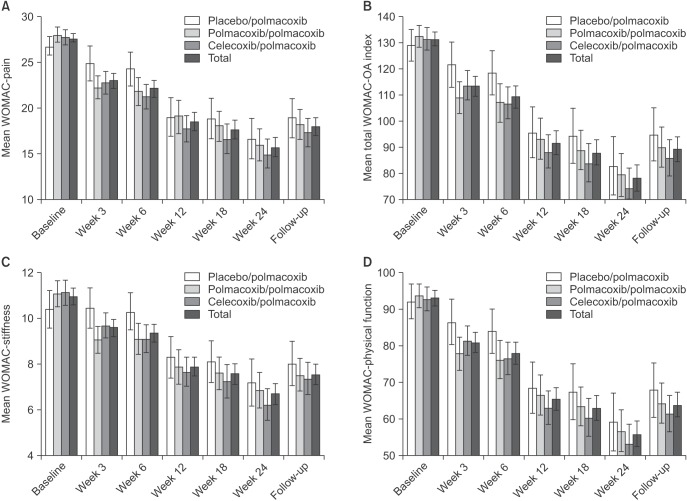
This study was a 6-week, phase III, randomized, double-blind, and parallel-group trial followed by an 18-week, single arm, open-label extension. Of the 441 patients with knee or hip OA screened, 362 were randomized; 324 completed 6 weeks of treatment and 220 completed the extension. Patients were randomized to receive oral polmacoxib 2 mg (n = 146), celecoxib 200 mg (n = 145), or placebo (n = 71) once daily for 6 weeks. During the extension, all participants received open-label polmacoxib 2 mg. The primary endpoint was the change in Western Ontario and McMaster Universities (WOMAC)-pain subscale score from baseline to week 6. Secondary endpoints included WOMAC-OA Index, OA subscales (pain, stiffness, and physical function) and Physician's and Subject's Global Assessments at weeks 3 and 6. Other outcome measures included adverse events (AEs), laboratory tests, vital signs, electrocardiograms, and physical examinations.
RESULTS
After 6 weeks, the polmacoxib-placebo treatment difference was −2.5 (95% confidence interval [CI], −4.4 to −0.6; p = 0.011) and the polmacoxib-celecoxib treatment difference was 0.6 (CI, −0.9 to 2.2; p = 0.425). According to Physician's Global Assessments, more subjects were "much improved" at week 3 with polmacoxib than with celecoxib or placebo. Gastrointestinal and general disorder AEs occurred with a greater frequency with polmacoxib or celecoxib than with placebo.
CONCLUSIONS
Polmacoxib 2 mg was relatively well tolerated and demonstrated efficacy superior to placebo and noninferior to celecoxib after 6 weeks of treatment in patients with OA. The results obtained during the 18-week trial extension with polmacoxib 2 mg were consistent with those observed during the 6-week treatment period, indicating that polmacoxib can be considered safe for long-term use based on this relatively small scale of study in a Korean population. More importantly, the results of this study showed that polmacoxib has the potential to be used as a pain relief drug with reduced gastrointestinal side effects compared to traditional nonsteroidal anti-inflammatory drugs for OA.
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Celecoxib/adverse effects/*therapeutic use
Cyclooxygenase 2 Inhibitors/adverse effects/*therapeutic use
Double-Blind Method
Female
Furans/adverse effects/*therapeutic use
Gastrointestinal Diseases/chemically induced
Humans
Male
Middle Aged
Musculoskeletal Pain/*drug therapy/etiology
Osteoarthritis, Hip/complications/*drug therapy/physiopathology
Osteoarthritis, Knee/complications/*drug therapy/physiopathology
Range of Motion, Articular
Sulfonamides/adverse effects/*therapeutic use
Cyclooxygenase 2 Inhibitors
Furans
Sulfonamides
Celecoxib
Figure
Reference
-
1. Poole AR. Osteoarthritis as a whole joint disease. HSS J. 2012; 8(1):4–6. PMID: 23372516.
Article2. Barbour KE, Helmick CG, Boring M, Brady TJ. Vital signs: prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation: United States, 2013-2015. MMWR Morb Mortal Wkly Rep. 2017; 66(9):246–253. PMID: 28278145.3. Hochberg MC, Altman RD, April KT, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012; 64(4):465–474. PMID: 22563589.
Article4. Garcia Rodriguez LA, Jick H. Risk of upper gastrointestinal bleeding and perforation associated with individual non-steroidal anti-inflammatory drugs. Lancet. 1994; 343(8900):769–772. PMID: 7907735.5. FitzGerald GA, Patrono C. The coxibs, selective inhibitors of cyclooxygenase-2. N Engl J Med. 2001; 345(6):433–442. PMID: 11496855.
Article6. Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev. 2004; 56(3):387–437. PMID: 15317910.
Article7. Turini ME, DuBois RN. Cyclooxygenase-2: a therapeutic target. Annu Rev Med. 2002; 53:35–57. PMID: 11818462.
Article8. Silverstein FE, Faich G, Goldstein JL, et al. Gastrointestinal toxicity with celecoxib vs nonsteroidal anti-inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study. A randomized controlled trial: celecoxib long-term arthritis safety study. JAMA. 2000; 284(10):1247–1255. PMID: 10979111.9. Bombardier C, Laine L, Reicin A, et al. VIGOR study group. Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. N Engl J Med. 2000; 343(21):1520–1528. PMID: 11087881.
Article10. Graham DJ, Campen D, Hui R, et al. Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: nested case-control study. Lancet. 2005; 365(9458):475–481. PMID: 15705456.
Article11. McGettigan P, Henry D. Cardiovascular risk and inhibition of cyclooxygenase: a systematic review of the observational studies of selective and nonselective inhibitors of cyclooxygenase 2. JAMA. 2006; 296(13):1633–1644. PMID: 16968831.12. Hawker GA, Katz JN, Solomon DH. The patient's perspective on the recall of Vioxx. J Rheumatol. 2006; 33(6):1082–1088. PMID: 16755654.13. Schmidt WK, Lehnhardt K, Hettwer J, et al. CG100649, a tissue-specific dual inhibitor of COX-2 and carbonic anhydrase: phase 2A clinical trial in hip & knee osteoarthritis. Osteoarthritis Cartilage. 2009; 17(Supple 1):S173.14. Cabrera J, Schmidt WK, Cho JM, Ro S, Singh G. Pharmacokinetic/pharmacodynamic (PK/PD) analysis of CG100649, a dual COX-2 & carbonic anhydrase inhibitor, in primary osteoarthritis of the hip or knee. Ann Rheum Dis. 2010; 69(Suppl 3):291. (abstract THU0442). PMID: 19204014.15. Hirankarn S, Barrett JS, Alamuddin N, FitzGerald GA, Skarke C. GCG100649, a novel cyclooxygenase-2 inhibitor, exhibits a drug disposition profile in healthy volunteers compatible with high affinity to carbonic anhydrase-I/II: preliminary dose-exposure relationships to define clinical development strategies. Clin Pharmacol Drug Dev. 2013; 2(4):379–386. PMID: 27121942.
Article16. Kim HT, Cha H, Hwang KY. Structural insight into the inhibition of carbonic anhydrase by the COX-2-selective inhibitor polmacoxib (CG100649). Biochem Biophys Res Commun. 2016; 478(1):1–6. PMID: 27475498.
Article17. Tashian RE. The carbonic anhydrases: widening perspectives on their evolution, expression and function. Bioessays. 1989; 10(6):186–192. PMID: 2500929.
Article18. Skarke C, Alamuddin N, Lawson JA, Cen L, Propert KJ, Fitzgerald GA. Comparative impact on prostanoid biosynthesis of celecoxib and the novel nonsteroidal anti-inflammatory drug CG100649. Clin Pharmacol Ther. 2012; 91(6):986–993. PMID: 22278334.
Article19. Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986; 29(8):1039–1049. PMID: 3741515.20. Altman R, Alarcon G, Appelrouth D, et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum. 1991; 34(5):505–514. PMID: 2025304.
Article21. Hochberg MC, Chang RW, Dwosh I, Lindsey S, Pincus T, Wolfe F. The American College of Rheumatology 1991 revised criteria for the classification of global functional status in rheumatoid arthritis. Arthritis Rheum. 1992; 35(5):498–502. PMID: 1575785.
Article22. Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988; 15(12):1833–1840. PMID: 3068365.23. Bae SC, Lee HS, Yun HR, Kim TH, Yoo DH, Kim SY. Cross-cultural adaptation and validation of Korean Western Ontario and McMaster Universities (WOMAC) and Lequesne osteoarthritis indices for clinical research. Osteoarthritis Cartilage. 2001; 9(8):746–750. PMID: 11795994.
Article24. Lane NE, Hochberg MC, Nevitt MC, et al. OARSI clinical trials recommendations: design and conduct of clinical trials for hip osteoarthritis. Osteoarthritis Cartilage. 2015; 23(5):761–771. PMID: 25952347.
Article25. ICH Expert Working Group. ICH harmonised tripartite guideline: the extent of population exposure to assess clinical safety for drugs intended for long-term treatment of non-life-threatening conditions E1. Geneva: International Council for Harmonisation;1994.26. Center for Drug Evaluation and Research. Center for Biologics Evaluation and Research. Non-inferiority clinical trials: guidance for industry. Silver Spring, MD: Food and Drug Administration;2010.27. Gibofsky A, Williams GW, McKenna F, Fort JG. Comparing the efficacy of cyclooxygenase 2-specific inhibitors in treating osteoarthritis: appropriate trial design considerations and results of a randomized, placebo-controlled trial. Arthritis Rheum. 2003; 48(11):3102–3111. PMID: 14613272.
Article28. Knights KM, Mangoni AA, Miners JO. Non-selective nonsteroidal anti-inflammatory drugs and cardiovascular events: is aldosterone the silent partner in crime? Br J Clin Pharmacol. 2006; 61(6):738–740. PMID: 16722838.
Article29. Park NG, Kim WK, Shin DH, et al. Prevalence of osteoarthritis and rheumatoid arthritis in two communities in Korea. J Korean Rheum Assoc. 2003; 10(2):151–157.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Multicenter Study in Efficacy and Safety of Celecoxib in Patients with Rheumatoid Arthritis and Osteoarthritis
- First-line bevacizumab plus chemotherapy in Chinese patients with stage III/IV epithelial ovarian cancer, fallopian tube cancer or primary peritoneal cancer: a phase III randomized controlled trial
- Treatment of Knee Osteoarthritis with Ketoprofen(Ketotop): A Double-blind Placebo-controlled Randomized Trial
- The Comparison of the Efficacy and Adverse Drug Reaction ofCelecoxib and Diclofenac in the Treatment of Osteoarthritis in Korean Multicenter Trial
- Correspondence to editorial on “Safety and efficacy of HK-660S in patients with primary sclerosing cholangitis: A randomized double-blind phase 2a trial”