Korean J Radiol.
2018 Jun;19(3):498-507. 10.3348/kjr.2018.19.3.498.
Blood-Brain Barrier Disruption and Perivascular Beta-Amyloid Accumulation in the Brain of Aged Rats with Spontaneous Hypertension: Evaluation with Dynamic Contrast-Enhanced Magnetic Resonance Imaging
- Affiliations
-
- 1Department of Radiology, West China Hospital, Sichuan University, Chengdu 610041, China. gaofabao@yahoo.com
- 2Department of Radiology, the Affiliated Hospital of Southwest Medical University, Luzhou 646000, China.
- 3Department of Neurosurgery, West China Hospital, Sichuan University, Chengdu 610041, China.
- 4Mallinckrodt Institute of Radiology, Washington University School of Medicine, St. Louis 63110, MO, USA.
- KMID: 2410821
- DOI: http://doi.org/10.3348/kjr.2018.19.3.498
Abstract
OBJECTIVE
Whether blood-brain barrier (BBB) disruption induced by chronic spontaneous hypertension is associated with beta-amyloid (Aβ) accumulation in the brain remains poorly understood. The purpose of this study was to investigate the relationship between BBB disruption and Aβ influx and accumulation in the brain of aged rats with chronic spontaneous hypertension.
MATERIALS AND METHODS
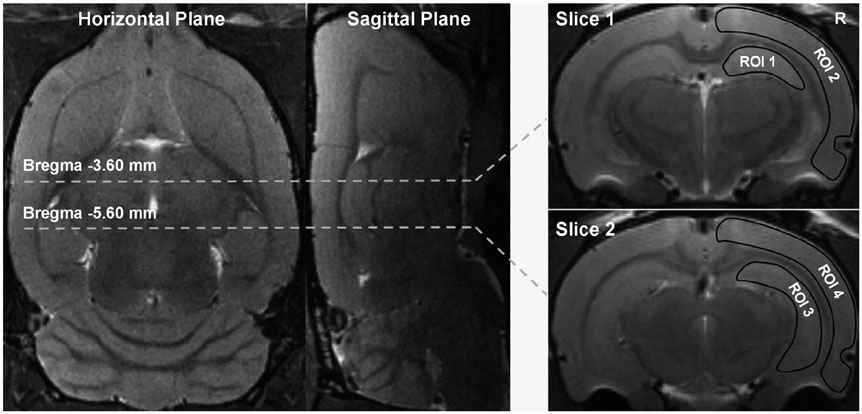
Five aged spontaneously hypertensive rats (SHRs) and five age-matched normotensive Wistar-Kyoto (WKY) rats were studied. The volume transfer constant (Ktrans) obtained from dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) was used to evaluate BBB permeability in the hippocampus and cortex in vivo. The BBB tight junctions, immunoglobulin G (IgG), Aβ, and amyloid precursor protein (APP) in the hippocampus and cortex were examined with immunohistochemistry.
RESULTS
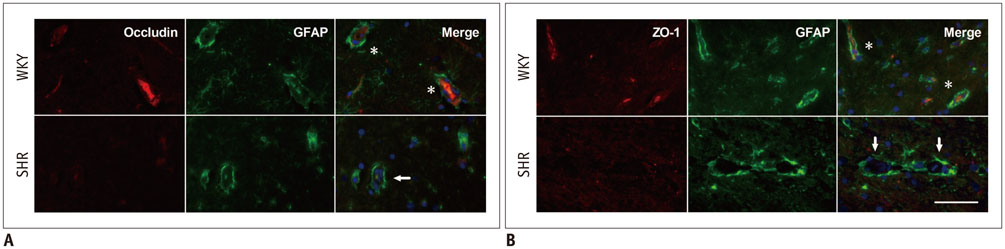
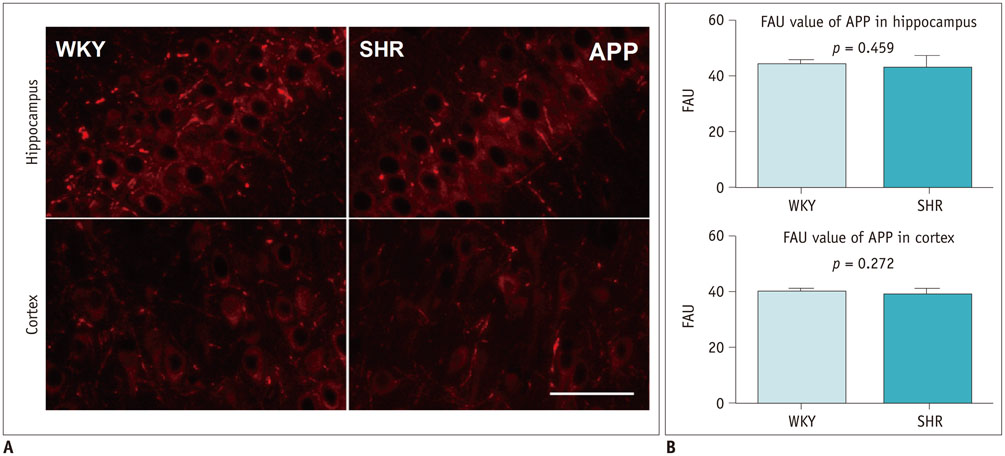
As compared with WKY rats, the Ktrans values in the hippocampus and cortex of the SHRs increased remarkably (0.316 ± 0.027 min−1 vs. 0.084 ± 0.017 min−1, p < 0.001 for hippocampus; 0.302 ± 0.072 min−1 vs. 0.052 ± 0.047 min−1, p < 0.001 for cortex). Dramatic occludin and zonula occludens-1 losses were detected in the hippocampus and cortex of SHRs, and obvious IgG exudation was found there. Dramatic Aβ accumulation was found and limited to the area surrounding the BBB, without extension to other parenchyma regions in the hippocampus and cortex of aged SHRs. Alternatively, differences in APP expression in the hippocampus and cortex were not significant.
CONCLUSION
Blood-brain barrier disruption is associated with Aβ influx and accumulation in the brain of aged rats with chronic spontaneous hypertension. DCE-MRI can be used as an effective method to investigated BBB damage.
MeSH Terms
Figure
Reference
-
1. Obermeier B, Daneman R, Ransohoff RM. Development, maintenance and disruption of the blood-brain barrier. NatMed. 2013; 19:1584–1596.
Article2. Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul Pharmacol. 2002; 38:323–337.3. Hawkins BT, Davis TP. The blood-brain barrier/neurovascular unit in health and disease. Pharmacol Rev. 2005; 57:173–185.
Article4. Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008; 57:178–201.
Article5. Pelisch N, Hosomi N, Mori H, Masaki T, Nishiyama A. RAS inhibition attenuates cognitive impairment by reducing blood- brain barrier permeability in hypertensive subjects. Curr Hypertens Rev. 2013; 9:93–98.
Article6. Ueno M, Sakamoto H, Tomimoto H, Akiguchi I, Onodera M, Huang CL, et al. Blood-brain barrier is impaired in the hippocampus of young adult spontaneously hypertensive rats. Acta Neuropathol. 2004; 107:532–538.
Article7. Miners JS, Baig S, Palmer J, Palmer LE, Kehoe PG, Love S. Abeta-degrading enzymes in Alzheimer's disease. Brain Pathol. 2008; 18:240–252.8. Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002; 297:353–356.
Article9. Nicolas M, Hassan BA. Amyloid precursor protein and neural development. Development. 2014; 141:2543–2548.
Article10. Wang W, Mutka AL, Zmrzljak UP, Rozman D, Tanila H, Gylling H, et al. Amyloid precursor protein α- and β-cleaved ectodomains exert opposing control of cholesterol homeostasis via SREBP2. FASEB J. 2014; 28:849–860.11. Bhatia R, Lin H, Lal R. Fresh and globular amyloid beta protein (1-42) induces rapid cellular degeneration: evidence for AbetaP channel-mediated cellular toxicity. FASEB J. 2000; 14:1233–1243.12. Bell RD, Zlokovic BV. Neurovascular mechanisms and bloodbrain barrier disorder in Alzheimer's disease. Acta Neuropathol. 2009; 118:103–113.
Article13. Zhang H, Ma Q, Zhang YW, Xu H. Proteolytic processing of Alzheimer's β-amyloid precursor protein. J Neurochem. 2012; 120:Suppl 1. 9–21.
Article14. Deane R, Du Yan S, Submamaryan RK, LaRue B, Jovanovic S, Hogg E, et al. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. NatMed. 2003; 9:907–913.15. Carnevale D, Mascio G, D'Andrea I, Fardella V, Bell RD, Branchi I, et al. Hypertension induces brain β-amyloid accumulation, cognitive impairment, and memory deterioration through activation of receptor for advanced glycation end products in brain vasculature. Hypertension. 2012; 60:188–197.
Article16. Hofman A, Ott A, Breteler MM, Bots ML, Slooter AJ, van Harskamp F, et al. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer's disease in the Rotterdam Study. Lancet. 1997; 349:151–154.
Article17. Schreiber S, Drukarch B, Garz C, Niklass S, Stanaszek L, Kropf S, et al. Interplay between age, cerebral small vessel disease, parenchymal amyloid-β, and tau pathology: longitudinal studies in hypertensive stroke-prone rats. J Alzheimers Dis. 2014; 42:Suppl 3. 205–215.
Article18. Faraco G, Park L, Zhou P, Luo W, Paul SM, Anrather J, et al. Hypertension enhances Aβ-induced neurovascular dysfunction, promotes β-secretase activity, and leads to amyloidogenic processing of APP. J Cereb Blood Flow Metab. 2016; 36:241–252.
Article19. Aksoy D, Bammer R, Mlynash M, Venkatasubramanian C, Eyngorn I, Snider RW, et al. Magnetic resonance imaging profile of blood-brain barrier injury in patients with acute intracerebral hemorrhage. J Am Heart Assoc. 2013; 2:e000161.
Article20. Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015; 85:296–302.
Article21. Ortuño JE, Ledesma-Carbayo MJ, Simões RV, Candiota AP, Arús C, Santos A. DCE@urLAB: a dynamic contrast-enhanced MRI pharmacokinetic analysis tool for preclinical data. BMC Bioinformatics. 2013; 14:316.
Article22. Heye AK, Culling RD, Valdés Hernández Mdel C, Thrippleton MJ, Wardlaw JM. Assessment of blood-brain barrier disruption using dynamic contrast-enhanced MRI. A systematic review. Neuroimage Clin. 2014; 6:262–274.
Article23. Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th ed. San Diego: Elsevier Academic Press;2007. p. 273–275.24. Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999; 10:223–232.
Article25. Nag S. Immunohistochemical detection of endothelial proteins. Methods Mol Med. 2003; 89:489–501.
Article26. Gentile MT, Poulet R, Di Pardo A, Cifelli G, Maffei A, Vecchione C, et al. Beta-amyloid deposition in brain is enhanced in mouse models of arterial hypertension. Neurobiol Aging. 2009; 30:222–228.27. Bailey EL, Wardlaw JM, Graham D, Dominiczak AF, Sudlow CL, Smith C. Cerebral small vessel endothelial structural changes predate hypertension in stroke-prone spontaneously hypertensive rats: a blinded, controlled immunohistochemical study of 5- to 21-week-old rats. Neuropathol Appl Neurobiol. 2011; 37:711–726.
Article28. Bueche CZ, Hawkes C, Garz C, Vielhaber S, Attems J, Knight RT, et al. Hypertension drives parenchymal β-amyloid accumulation in the brain parenchyma. Ann Clin Transl Neurol. 2014; 1:124–129.
Article29. Csiszar A, Tucsek Z, Toth P, Sosnowska D, Gautam T, Koller A, et al. Synergistic effects of hypertension and aging on cognitive function and hippocampal expression of genes involved in β-amyloid generation and Alzheimer's disease. AmJ Physiol Heart Circ Physiol. 2013; 305:1120–1130.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- beta-Amyloid Imaging Probes
- Postoperative Meningeal Enhancement on MRI in Children with Brain Neoplasms
- Alzheimer Dementia and Microvascular Pathology: Blood-Brain Barrier Permeability Imaging
- Is the Phenomenon of Blood-Brain Barrier Disruption Induced with Mannitol All-or-None?
- Advances in Amyloid-β Clearance in the Brain and Periphery: Implications for Neurodegenerative Diseases