J Rheum Dis.
2018 Apr;25(2):108-115. 10.4078/jrd.2018.25.2.108.
Transaminase Changes in Korean Rheumatoid Arthritis Patients with Chronic Hepatitis C after Biologic Therapy
- Affiliations
-
- 1Division of Rheumatology, Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea. ysong@snu.ac.kr
- 2Division of Rheumatology, Department of Internal Medicine, SMG-SNU Boramae Medical Center, Seoul, Korea.
- 3Division of Rheumatology, Department of Internal Medicine, Chonnam National University Hospital, Gwangju, Korea.
- 4Division of Rheumatology, Department of Internal Medicine, Dong-A University Hospital, Busan, Korea.
- 5Division of Rheumatology, Department of Internal Medicine, Ewha Womans University Mokdong Hospital, Seoul, Korea.
- 6Division of Rheumatology, Department of Internal Medicine, Konkuk University Medical Center, Seoul, Korea.
- 7Division of Rheumatology, Department of Internal Medicine, Chungnam National University Hospital, Daejeon, Korea.
- 8Division of Rheumatology, Department of Internal Medicine, Ajou University Hospital, Suwon, Korea.
- 9Division of Rheumatology, Department of Internal Medicine, Kyung Hee University Medical Center, Seoul, Korea.
- 10Division of Rheumatology, Department of Internal Medicine, Kyung Hee University Hospital at Gangdong, Seoul, Korea.
- 11Division of Rheumatology, Department of Internal Medicine, Daegu Catholic University Medical Center, Daegu, Korea.
- 12Department of Molecular Medicine and Biopharmaceutical Sciences, Graduate School of Convergence Science and Technology, and College of Medicine, Medical Research Center, Seoul National University, Seoul, Korea.
- KMID: 2408928
- DOI: http://doi.org/10.4078/jrd.2018.25.2.108
Abstract
OBJECTIVE
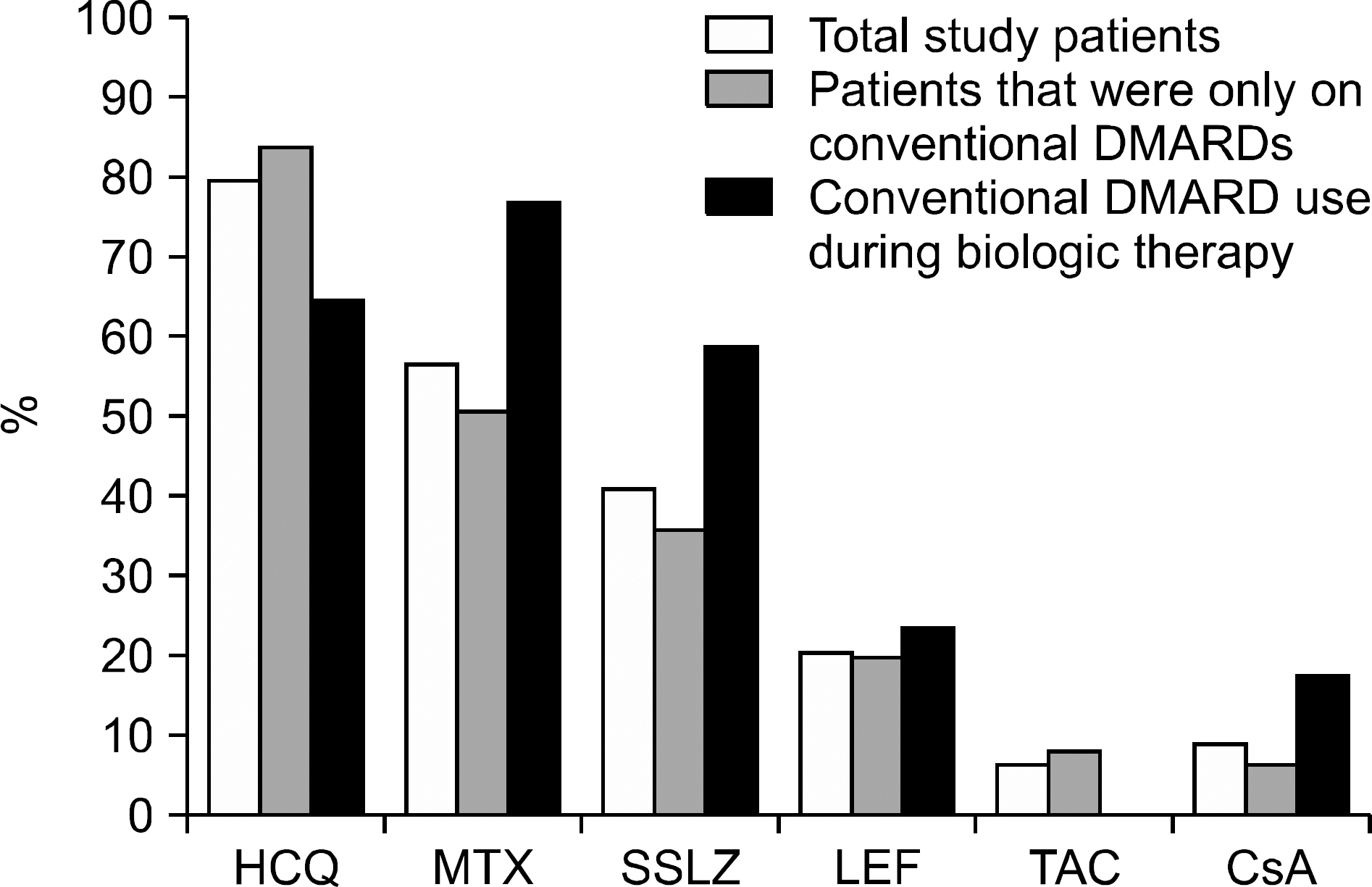
Coexisting chronic hepatitis C can be problematic when treating rheumatoid arthritis (RA). This study examined the changes in the transaminase and viral load in hepatitis C virus (HCV)-infected RA patients after initiating biologic agents.
METHODS
A multicenter retrospective study was conducted at 12 University Hospitals in Korea between November 2014 and November 2015, and 78 RA patients, who met the 2010 American College of Rheumatology and European League Against Rheumatism classification criteria for RA and were concomitantly infected with HCV, were identified. The baseline and longitudinal clinical data, changes in liver function, and viral RNA titers were evaluated.
RESULTS
Seventeen (21.8%) patients were treated with biologic agents, including etanercept (n=8), adalimumab (n=8), infliximab (n=2), tocilizumab (n=2), abatacept (n=1), and golimumab (n=1) (median 1.5 patient-years). Four patients experienced marked increases in transaminase during treatment with adalimumab (n=2) and tocilizumab (n=2). Two patients (one using adalimumab, the other using tocilizumab) were treated with anti-viral agents and showed dramatic improvement in both the viral RNA and transaminase. One patient discontinued adalimumab due to the repeated elevated transaminase levels along with a twofold increase in the viral RNA titer, and the transaminase level subsequently normalized. No case of overt viral reactivation was identified.
CONCLUSION
The data support that changes in transaminase and/or viral load associated with biologic agents in HCV-infected RA patients are possible. Therefore, the liver function and viral RNA titer should be followed regularly during biologic therapy.
MeSH Terms
-
Abatacept
Adalimumab
Antirheumatic Agents
Arthritis, Rheumatoid*
Biological Factors
Biological Therapy*
Classification
Etanercept
Hepacivirus
Hepatitis C
Hepatitis C, Chronic*
Hepatitis, Chronic*
Hospitals, University
Humans
Infliximab
Korea
Liver
Retrospective Studies
Rheumatic Diseases
Rheumatology
RNA, Viral
Viral Load
Abatacept
Adalimumab
Antirheumatic Agents
Biological Factors
Etanercept
Infliximab
RNA, Viral
Figure
Cited by 1 articles
-
Chronic Hepatitis C Virus Infection, Why Not Treat Now?
Kyung-Su Park
J Rheum Dis. 2018;25(3):151-152. doi: 10.4078/jrd.2018.25.3.151.
Reference
-
1. Bojito-Marrero L, Pyrsopoulos N. Hepatitis B and Hepatitis C reactivation in the biologic Era. J Clin Transl Hepatol. 2014; 2:240–6.
Article2. Yazici O, Sendur MA, Aksoy S. Hepatitis C virus reactivation in cancer patients in the era of targeted therapies. World J Gastroenterol. 2014; 20:6716–24.
Article3. Yeo W, Chan TC, Leung NW, Lam WY, Mo FK, Chu MT, et al. Hepatitis B virus reactivation in lymphoma patients with prior resolved hepatitis B undergoing anticancer therapy with or without rituximab. J Clin Oncol. 2009; 27:605–11.
Article4. Pérez-Alvarez R, Díaz-Lagares C, García-Hernández F, Lopez-Roses L, Brito-Zerón P, Pérez-de-Lis M, et al. Hepatitis B virus (HBV) reactivation in patients receiving tumor necrosis factor (TNF)-targeted therapy: analysis of 257 cases. Medicine (Baltimore). 2011; 90:359–71.5. Lau GK, Leung YH, Fong DY, Au WY, Kwong YL, Lie A, et al. High hepatitis B virus (HBV) DNA viral load as the most important risk factor for HBV reactivation in patients positive for HBV surface antigen undergoing autologous hematopoietic cell transplantation. Blood. 2002; 99:2324–30.
Article6. Nathan DM, Angus PW, Gibson PR. Hepatitis B and C virus infections and anti-tumor necrosis factor-alpha therapy: guidelines for clinical approach. J Gastroenterol Hepatol. 2006; 21:1366–71.7. National Institutes of Health Consensus Development Conference Panel statement: management of hepatitis C. Hepatology. 1997; 26(3 Suppl 1):2S–10S.8. Torres HA, Davila M. Reactivation of hepatitis B virus and hepatitis C virus in patients with cancer. Nat Rev Clin Oncol. 2012; 9:156–66.
Article9. Mahale P, Kontoyiannis DP, Chemaly RF, Jiang Y, Hwang JP, Davila M, et al. Acute exacerbation and reactivation of chronic hepatitis C virus infection in cancer patients. J Hepatol. 2012; 57:1177–85.
Article10. Vento S, Cainelli F, Mirandola F, Cosco L, Di Perri G, Solbiati M, et al. Fulminant hepatitis on withdrawal of chemotherapy in carriers of hepatitis C virus. Lancet. 1996; 347:92–3.
Article11. Lawrence RC, Hochberg MC, Kelsey JL, McDuffie FC, Medsger TA Jr, Felts WR, et al. Estimates of the prevalence of selected arthritic and musculoskeletal diseases in the United States. J Rheumatol. 1989; 16:427–41.12. Maillefert JF, Muller G, Falgarone G, Bour JB, Ratovohery D, Dougados M, et al. Prevalence of hepatitis C virus infection in patients with rheumatoid arthritis. Ann Rheum Dis. 2002; 61:635–7.
Article13. Brunasso AM, Puntoni M, Gulia A, Massone C. Safety of an-ti-tumour necrosis factor agents in patients with chronic hepatitis C infection: a systematic review. Rheumatology (Oxford). 2011; 50:1700–11.
Article14. Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/ European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010; 62:2569–81.15. Nguyen TT, Sedghi-Vaziri A, Wilkes LB, Mondala T, Pockros PJ, Lindsay KL, et al. Fluctuations in viral load (HCV RNA) are relatively insignificant in untreated patients with chronic HCV infection. J Viral Hepat. 1996; 3:75–8.16. Fanning L, Kenny-Walsh E, Levis J, Choudhury KR, Cannon B, Sheehan M, et al. Natural fluctuations of hepatitis C viral load in a homogeneous patient population: a prospective study. Hepatology. 2000; 31:225–9.
Article17. Iannone F, Gremese E, Ferraccioli G, Lapadula G; GISEA. Comment on “Tumor necrosis factor-α antagonist therapy for concomitant rheumatoid arthritis and hepatitis C virus infection: a case series study”. Clin Rheumatol. 2016; 35:839–40.
Article18. Temel T, Cansu DÜ, Korkmaz C, Kaş ifoğlu T, Özakyol A. The long-term effects of anti-TNF-α agents on patients with chronic viral hepatitis C and B infections. Int J Rheum Dis. 2015; 18:40–5.
Article19. Lin KM, Cheng TT, Lin JC, Chen CJ. Tumor necrosis factor-α antagonist therapy for concomitant rheumatoid arthritis and hepatitis C virus infection: a case series study. Clin Rheumatol. 2015; 34:1039–46.
Article20. Iannone F, La Montagna G, Bagnato G, Gremese E, Giardina A, Lapadula G. Safety of etanercept and methotrexate in patients with rheumatoid arthritis and hepatitis C virus infection: a multicenter randomized clinical trial. J Rheumatol. 2014; 41:286–92.
Article21. Ballanti E, Conigliaro P, Chimenti MS, Kroegler B, Di Muzio G, Guarino MD, et al. Use of anti-tumor necrosis factor alpha therapy in patients with concurrent rheumatoid arthritis and hepatitis B or hepatitis C: a retrospective analysis of 32 patients. Drug Dev Res. 2014; 75(Suppl 1):S42–5.
Article22. Pompili M, Biolato M, Miele L, Grieco A. Tumor necrosis factor-α inhibitors and chronic hepatitis C: a comprehensive literature review. World J Gastroenterol. 2013; 19:7867–73.
Article23. Li S, Kaur PP, Chan V, Berney S. Use of tumor necrosis factor-alpha (TNF-alpha) antagonists infliximab, etanercept, and adalimumab in patients with concurrent rheumatoid arthritis and hepatitis B or hepatitis C: a retrospective record review of 11 patients. Clin Rheumatol. 2009; 28:787–91.24. Ferri C, Ferraccioli G, Ferrari D, Galeazzi M, Lapadula G, Montecucco C, et al. Safety of anti-tumor necrosis factor-alpha therapy in patients with rheumatoid arthritis and chronic hepatitis C virus infection. J Rheumatol. 2008; 35:1944–9.26. Calabrese LH, Zein N, Vassilopoulos D. Safety of anti-tumour necrosis factor (anti-TNF) therapy in patients with chronic viral infections: hepatitis C, hepatitis B, and HIV infection. Ann Rheum Dis. 2004; 63(Suppl 2):ii18–ii24.
Article27. Peterson JR, Hsu FC, Simkin PA, Wener MH. Effect of tumour necrosis factor alpha antagonists on serum transaminases and viraemia in patients with rheumatoid arthritis and chronic hepatitis C infection. Ann Rheum Dis. 2003; 62:1078–82.28. Pritchard C. Etanercept and hepatitis C. J Clin Rheumatol. 1999; 5:179.
Article29. Nagashima T, Maruyama A, Kamata Y, Minota S. Unchanged serum viral load and liver function during tocilizumab treatment in a patient with rheumatoid arthritis and hepatitis C virus infection. Rheumatol Int. 2012; 32:2231–2.
Article30. Dragonas C, Ehrenstein B, Fleck M. Tocilizumab treatment in a patient suffering from rheumatoid arthritis and concomitant chronic hepatitis C infection. Rheumatology (Oxford). 2012; 51:1520–1.
Article31. Mori S, Fujiyama S. Comment on: Tocilizumab treatment in a patient suffering from rheumatoid arthritis and concomitant chronic hepatitis C infection. Rheumatology (Oxford). 2012; 51:2300–2.
Article32. Streetz KL, Luedde T, Manns MP, Trautwein C. Interleukin 6 and liver regeneration. Gut. 2000; 47:309–12.
Article33. Eshbaugh M, Zito S. Abatacept therapy for rheumatoid arthritis in patients with hepatitis C virus infection comorbidity: a series of four patients. Rheumatology (Sunnyvale). 2014; S16:007.
Article34. Tsutsumi Y, Yamamoto Y, Ito S, Ohigashi H, Shiratori S, Naruse H, et al. Hepatitis B virus reactivation with a rituximab-containing regimen. World J Hepatol. 2015; 7:2344–51.
Article35. Chen YM, Chen HH, Chen YH, Hsieh TY, Hsieh CW, Hung WT, et al. A comparison of safety profiles of tumour necrosis factor α inhibitors and rituximab therapy in patients with rheumatoid arthritis and chronic hepatitis C. Ann Rheum Dis. 2015; 74:626–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Anti-Cyclic Citrullinated Peptide Antibody-Positive Rheumatoid Arthritis Following Peginterferon Alpha-2A and Ribavirin Therapy for Chronic Hepatitis C
- Toward the cure of rheumatoid arthritis
- Cytokines in rheumatoid arthritis
- Injectable gold-induced hepatitis and neutropenia in rheumatoid arthritis
- Medical Treatment of Rheumatoid Arthritis