Clin Exp Otorhinolaryngol.
2018 Mar;11(1):17-22. 10.21053/ceo.2017.00304.
Expression of Prostaglandin E2 Receptors in Acquired Middle Ear Cholesteatoma
- Affiliations
-
- 1Department of Otolaryngology-Head and Neck Surgery, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China. aiguoliu309@163.com
- 2Department of Pathology, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
- KMID: 2407787
- DOI: http://doi.org/10.21053/ceo.2017.00304
Abstract
OBJECTIVES
To investigate the expression of prostaglandin E2 receptor subtypes, E-prostanoid (EP) 1-4 receptors, in acquired cholesteatoma and its possible role in the pathologic process of this disorder.
METHODS
Specimens of human acquired cholesteatoma were obtained from 29 patients and 19 skin biopsies of normal external auditory canal were as controls. The mRNA and protein expression of EP receptors was assessed by quantitative real-time polymerase chain reaction, immunohistochemistry and Western blot.
RESULTS
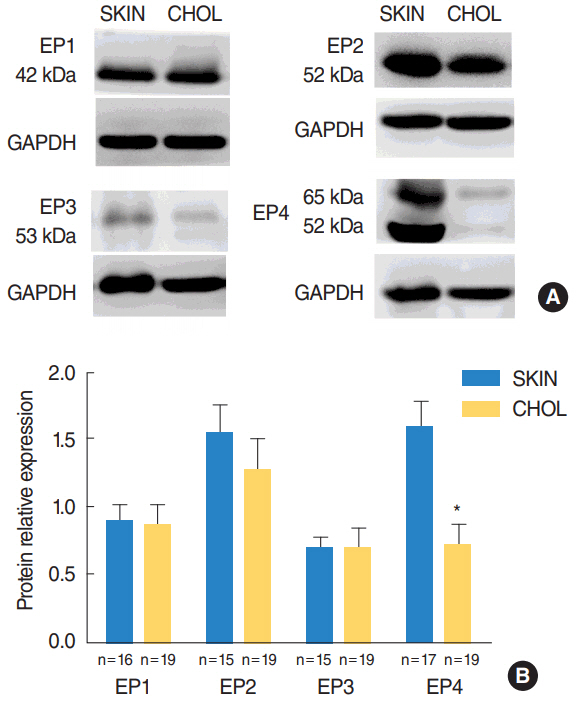
In acquired cholesteatoma, EP1-EP4 receptors were mainly expressed on squamous epithelium and subepithelial infiltrated inflammatory cells. In external auditory canal skin, EP1-EP4 receptors were mainly expressed on squamous epithelium and glandular epithelium. The expression of EP4 receptor on mRNA and protein levels were significant lower in acquired cholesteatoma compared with controls. EP1-EP3 receptors had no significant difference between the experimental and control group.
CONCLUSION
Low expression of EP4 may play a crucial role in the pathologic process of inflammation reaction and bone destruction in acquired cholesteatoma, but not EP1, EP2, or EP3 receptors.
MeSH Terms
Figure
Reference
-
1. Olszewska E, Wagner M, Bernal-Sprekelsen M, Ebmeyer J, Dazert S, Hildmann H, et al. Etiopathogenesis of cholesteatoma. Eur Arch Otorhinolaryngol. 2004; Jan. 261(1):6–24.
Article2. Juhn SK, Jung MK, Hoffman MD, Drew BR, Preciado DA, Sausen NJ, et al. The role of inflammatory mediators in the pathogenesis of otitis media and sequelae. Clin Exp Otorhinolaryngol. 2008; Sep. 1(3):117–38.
Article3. Liu W, Xie S, Chen X, Rao X, Ren H, Hu B, et al. Activation of the IL-6/JAK/STAT3 signaling pathway in human middle ear cholesteatoma epithelium. Int J Clin Exp Pathol. 2014; Jan. 7(2):709–15.4. Sakuma Y, Tanaka K, Suda M, Yasoda A, Natsui K, Tanaka I, et al. Crucial involvement of the EP4 subtype of prostaglandin E receptor in osteoclast formation by proinflammatory cytokines and lipopolysaccharide. J Bone Miner Res. 2000; Feb. 15(2):218–27.
Article5. Jung TT, Juhn SK. Prostaglandins in human cholesteatoma and granulation tissue. Am J Otol. 1988; May. 9(3):197–200.6. Shirahata Y, Abramson M. Experimental aural cholesteatoma causing bone resorption. Nihon Jibiinkoka Gakkai Kaiho. 1981; Nov. 84(11):1451–9.7. Hristovska AM, Rasmussen LE, Hansen PB, Nielsen SS, Nusing RM, Narumiya S, et al. Prostaglandin E2 induces vascular relaxation by E-prostanoid 4 receptor-mediated activation of endothelial nitric oxide synthase. Hypertension. 2007; Sep. 50(3):525–30.8. Yokoyama U, Minamisawa S, Quan H, Ghatak S, Akaike T, Segi-Nishida E, et al. Chronic activation of the prostaglandin receptor EP4 promotes hyaluronan-mediated neointimal formation in the ductus arteriosus. J Clin Invest. 2006; Nov. 116(11):3026–34.
Article9. Takayama K, Garcia-Cardena G, Sukhova GK, Comander J, Gimbrone MA Jr, Libby P. Prostaglandin E2 suppresses chemokine production in human macrophages through the EP4 receptor. J Biol Chem. 2002; Nov. 277(46):44147–54.
Article10. Kabashima K, Saji T, Murata T, Nagamachi M, Matsuoka T, Segi E, et al. The prostaglandin receptor EP4 suppresses colitis, mucosal damage and CD4 cell activation in the gut. J Clin Invest. 2002; Apr. 109(7):883–93.
Article11. Nitta M, Hirata I, Toshina K, Murano M, Maemura K, Hamamoto N, et al. Expression of the EP4 prostaglandin E2 receptor subtype with rat dextran sodium sulphate colitis: colitis suppression by a selective agonist, ONO-AE1-329. Scand J Immunol. 2002; Jul. 56(1):66–75.
Article12. Jee WS, Ueno K, Deng YP, Woodbury DM. The effects of prostaglandin E2 in growing rats: increased metaphyseal hard tissue and cortico-endosteal bone formation. Calcif Tissue Int. 1985; Mar. 37(2):148–57.
Article13. Jee WS, Ueno K, Kimmel DB, Woodbury DM, Price P, Woodbury LA. The role of bone cells in increasing metaphyseal hard tissue in rapidly growing rats treated with prostaglandin E2. Bone. 1987; 8(3):171–8.
Article14. Li XJ, Jee WS, Li YL, Patterson-Buckendahl P. Transient effects of subcutaneously administered prostaglandin E2 on cancellous and cortical bone in young adult dogs. Bone. 1990; 11(5):353–64.
Article15. Chyun YS, Raisz LG. Stimulation of bone formation by prostaglandin E2. Prostaglandins. 1984; Jan. 27(1):97–103.
Article16. Nagata T, Kaho K, Nishikawa S, Shinohara H, Wakano Y, Ishida H. Effect of prostaglandin E2 on mineralization of bone nodules formed by fetal rat calvarial cells. Calcif Tissue Int. 1994; Dec. 55(6):451–7.
Article17. Alander CB, Raisz LG. Effects of selective prostaglandins E2 receptor agonists on cultured calvarial murine osteoblastic cells. Prostaglandins Other Lipid Mediat. 2006; Dec. 81(3-4):178–83.
Article18. Shamir D, Keila S, Weinreb M. A selective EP4 receptor antagonist abrogates the stimulation of osteoblast recruitment from bone marrow stromal cells by prostaglandin E2 in vivo and in vitro. Bone. 2004; Jan. 34(1):157–62.
Article19. Weinreb M, Shamir D, Machwate M, Rodan GA, Harada S, Keila S. Prostaglandin E2 (PGE2) increases the number of rat bone marrow osteogenic stromal cells (BMSC) via binding the EP4 receptor, activating sphingosine kinase and inhibiting caspase activity. Prostaglandins Leukot Essent Fatty Acids. 2006; Aug. 75(2):81–90.
Article20. Machwate M, Harada S, Leu CT, Seedor G, Labelle M, Gallant M, et al. Prostaglandin receptor EP(4) mediates the bone anabolic effects of PGE(2). Mol Pharmacol. 2001; Jul. 60(1):36–41.
Article21. Lader CS, Flanagan AM. Prostaglandin E2, interleukin 1alpha, and tumor necrosis factor-alpha increase human osteoclast formation and bone resorption in vitro. Endocrinology. 1998; Jul. 139(7):3157–64.22. Sarrazin P, Bkaily G, Hache R, Patry C, Dumais R, Rocha FA, et al. Characterization of the prostaglandin receptors in human osteoblasts in culture. Prostaglandins Leukot Essent Fatty Acids. 2001; Mar. 64(3):203–10.
Article23. Mano M, Arakawa T, Mano H, Nakagawa M, Kaneda T, Kaneko H, et al. Prostaglandin E2 directly inhibits bone-resorbing activity of isolated mature osteoclasts mainly through the EP4 receptor. Calcif Tissue Int. 2000; Jul. 67(1):85–92.
Article24. Yoshida K, Oida H, Kobayashi T, Maruyama T, Tanaka M, Katayama T, et al. Stimulation of bone formation and prevention of bone loss by prostaglandin E EP4 receptor activation. Proc Natl Acad Sci U S A. 2002; Apr. 99(7):4580–5.
Article25. Iino Y, Toriyama M, Ogawa H, Kawakami M. Cholesteatoma debris as an activator of human monocytes: potentiation of the production of tumor necrosis factor. Acta Otolaryngol. 1990; Nov-Dec. 110(5-6):410–5.
Article26. Xie L, Liu AG, Cui YH, Zhang YP, Liao B, Li NN, et al. Expression profiles of prostaglandin E2 receptor subtypes in aspirin tolerant adult Chinese with chronic rhinosinusitis. Am J Rhinol Allergy. 2015; Sep-Oct. 29(5):322–8.
Article27. Morimoto K, Shirata N, Taketomi Y, Tsuchiya S, Segi-Nishida E, Inazumi T, et al. Prostaglandin E2-EP3 signaling induces inflammatory swelling by mast cell activation. J Immunol. 2014; Feb. 192(3):1130–7.
Article28. Sakanaka M, Tanaka S, Sugimoto Y, Ichikawa A. Essential role of EP3 subtype in prostaglandin E2-induced adhesion of mouse cultured and peritoneal mast cells to the Arg-Gly-Asp-enriched matrix. Am J Physiol Cell Physiol. 2008; Nov. 295(5):C1427–33.
Article29. Minamizaki T, Yoshiko Y, Kozai K, Aubin JE, Maeda N. EP2 and EP4 receptors differentially mediate MAPK pathways underlying anabolic actions of prostaglandin E2 on bone formation in rat calvaria cell cultures. Bone. 2009; Jun. 44(6):1177–85.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Incudal Osteoma Accompanied by Primary Acquired Cholesteatoma
- A Case of Two Isolated Congenital Cholesteatomas Presented in Middle Ear Cavity
- Prostaglandin E2 receptors as therapeutic targets in renal fibrosis
- Expression of Toll-Like Receptor 2 and Toll-Like Receptor 4 in Cholesteatoma
- A Retroauricular Cholesteatoma in Soft Tissue after Tympanomastoidectomy