Blood Res.
2017 Dec;52(4):307-310. 10.5045/br.2017.52.4.307.
Is long term storage of cryopreserved stem cells for hematopoietic stem cell transplantation a worthwhile exercise in developing countries?
- Affiliations
-
- 1Department of Medical Oncology and Bone Marrow Transplantation, Tata Memorial Center, Advanced Center for Treatment, Research and Education in Cancer (ACTREC), Mumbai, India. nkhattry@gmail.com
- 2Department of Transfusion Medicine, Tata Memorial Center, Advanced Center for Treatment, Research and Education in Cancer (ACTREC), Mumbai, India.
- 3Statistics, Tata Memorial Center, Advanced Center for Treatment, Research and Education in Cancer (ACTREC), Mumbai, India.
- KMID: 2405068
- DOI: http://doi.org/10.5045/br.2017.52.4.307
Abstract
- BACKGROUND
Stem cell units (SCUs) that are cryopreserved prior to both autologous and allogeneic hematopoietic stem cell transplants (for donor lymphocyte infusion) remain unused or partially used several times, and become an increased burden to blood banks/SCU repositories. Because of the scarcity of data regarding the duration for which the storage is useful, there is no general consensus regarding disposal of SCUs.
METHODS
We conducted a retrospective audit of SCU utilization in 435 patients who planned to undergo either autologous stem cell transplantation (auto-SCT) (N=239) or allogeneic stem cell transplantation (allo-SCT) (N=196) at a tertiary cancer care center between November 2007 to January 2015.
RESULTS
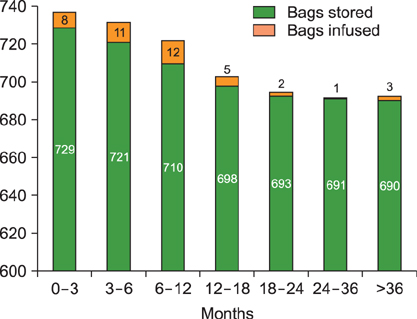
Our cohort consisted of 1,728 SCUs stored for conducting auto-SCT and 729 SCUs stored for conducting donor lymphocyte infusions (DLIs) after allo-SCT. Stem cells were not infused in 12.5% of patients who had planned to undergo auto-SCT, and 80% of patients who underwent allo-SCT never received DLI. Forty-one percent of SCUs intended for use in auto-SCT remained unutilized, with a second auto-SCT being performed only in 4 patients. Ninety-four percent of SCUs intended for carrying out DLIs remained unused, with only minimal usage observed one year after undergoing allo-SCT.
CONCLUSION
The duration of storage of unused SCUs needs to be debated upon, so that a consensus can be reached regarding the ethical disposal of SCU.
Keyword
MeSH Terms
Figure
Reference
-
1. Lennard AL, Jackson GH. Stem cell transplantation. BMJ. 2000; 321:433–437.2. Frey NV, Lazarus HM, Goldstein SC. Has allogeneic stem cell cryopreservation been given the ‘cold shoulder’? An analysis of the pros and cons of using frozen versus fresh stem cell products in allogeneic stem cell transplantation. Bone Marrow Transplant. 2006; 38:399–405.
Article3. Bladé J, Rosiñol L, Fernández de. How I treat relapsed myeloma. Blood. 2015; 125:1532–1540.
Article4. Ritchie DS. The role of second autografts in the treatment of Hodgkin lymphoma. Leuk Lymphoma. 2007; 48:847–848.
Article5. Kayano D, Kinuya S. Iodine-131 metaiodobenzylguanidine therapy for neuroblastoma: reports so far and future perspective. ScientificWorldJournal. 2015; 2015:189135.
Article6. European Society for Blood and Marrow Transplantation. EBMT Annual report 2014. Leiden, The Netherlands: EBMT;2015. Accessed February 4, 2017. https://www.ebmt.org/Contents/Resources/Library/Annualreport/Documents/EBMT_AnnualReport_2014.pdf.7. Mohty M, Harousseau JL. Treatment of autologous stem cell transplant-eligible multiple myeloma patients: ten questions and answers. Haematologica. 2014; 99:408–416.
Article8. Attal M, Harousseau JL, Facon T, et al. Single versus double autologous stem-cell transplantation for multiple myeloma. N Engl J Med. 2003; 349:2495–2502.
Article9. Singh Abbi KK, Zheng J, Devlin SM, Giralt S, Landau H. Second autologous stem cell transplant: an effective therapy for relapsed multiple myeloma. Biol Blood Marrow Transplant. 2015; 21:468–472.
Article10. Morschhauser F, Brice P, Fermé C, et al. Risk-adapted salvage treatment with single or tandem autologous stem-cell transplantation for first relapse/refractory Hodgkin's lymphoma: results of the prospective multicenter H96 trial by the GELA/SFGM study group. J Clin Oncol. 2008; 26:5980–5987.
Article11. Olivieri J, Pierelli L, Introna M, et al. Kinetics of the use of cryopreserved autologous stem cell grafts: a GITMO-SIDEM survey. Cytotherapy. 2014; 16:101–110.
Article12. Chang YJ, Huang XJ. Donor lymphocyte infusions for relapse after allogeneic transplantation: when, if and for whom? Blood Rev. 2013; 27:55–62.
Article13. Bakken R, van Walraven AM, Egeland T. Ethics Working Group of the World Marrow Donor Association. Donor commitment and patient needs. Bone Marrow Transplant. 2004; 33:225–230.
Article14. FACT-JACIE Hematopoietic Cellular Therapy Accreditation Manual. 6th ed. Omaha, NE: The Foundation for the Accreditation of Cellular Therapy;2017. p. 434–435.15. Jaime-Perez JC, Monreal-Robles R, Colunga-Pedraza J, Mancías-Guerra C, Rodríguez-Romo L, Gómez-Almaguer D. Cord blood banking activities at a university hospital in northeast Mexico: an 8-year experience. Transfusion. 2012; 52:2606–2613.
Article16. Mishra V, Andresen S, Brinch L, et al. Cost of autologous peripheral blood stem cell transplantation: the Norwegian experience from a multicenter cost study. Bone Marrow Transplant. 2005; 35:1149–1153.
Article17. Shaughnessy P, Islas-Ohlmayer M, Murphy J, et al. Cost and clinical analysis of autologous hematopoietic stem cell mobilization with G-CSF and plerixafor compared to G-CSF and cyclophosphamide. Biol Blood Marrow Transplant. 2011; 17:729–736.
Article18. Cameron G, Tantiworawit A, Halpenny M, et al. Cryopreserved mobilized autologous blood progenitors stored for more than 2 years successfully support blood count recovery after high-dose chemotherapy. Cytotherapy. 2011; 13:856–863.
Article19. Khera N, Zeliadt SB, Lee SJ. Economics of hematopoietic cell transplantation. Blood. 2012; 120:1545–1551.
Article20. Sharma SK, Choudhary D, Gupta N, et al. Cost of hematopoietic stem cell transplantation in India. Mediterr J Hematol Infect Dis. 2014; 6:e2014046.
Article21. Lee SJ, Klar N, Weeks JC, Antin JH. Predicting costs of stem-cell transplantation. J Clin Oncol. 2000; 18:64–71.22. Perseghin P, Marchetti M, Pierelli L, et al. A policy for the disposal of autologous hematopoietic progenitor cells: report from an Italian consensus panel. Transfusion. 2014; 54:2353–2360.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hematopoietic stem cell transplantation: overview for general pediatrician
- Comparison of Hematopoietic Stem Cell Containing Fractions between Cryopreserved-thawed Cord Blood and Mobilized Peripheral Blood
- Opening the era of in vivo xenotransplantation model for hematopoietic stem cell transplantation
- Hematopoietic Stem Cell Transplantation
- The Fetal Sheep: A Unique Model System for Assessing the Full Differentiative Potential of Human Stem Cells