Cancer Res Treat.
2018 Jan;50(1):30-39. 10.4143/crt.2016.569.
S-1–Induced Lacrimal Drainage Obstruction and Its Association with Ingredients/Metabolites of S-1 in Tears and Plasma: A Prospective Multi-institutional Study
- Affiliations
-
- 1Department of Ophthalmology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 2Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea. hmodoctor@snubh.org
- 3Center of Biomedical Mass Spectrometry, Diatech Korea Co., Ltd., Seoul, Korea.
- 4Department of Internal Medicine, Seoul National University Boramae Medical Center, Seoul, Korea.
- 5Department of Ophthalmology, Seoul National University Boramae Medical Center, Seoul, Korea.
- 6Department of Internal Medicine, Seoul National University College of Medicine, Seoul National University Hospital, Seoul, Korea.
- 7Department of Ophthalmology, Seoul National University College of Medicine, Seoul National University Hospital, Seoul, Korea.
- 8Department of Surgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 9Department of Clinical Pharmacology and Therapeutics, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea.
- 10Medical Research Collaborating Center, Seoul National University Bundang Hospital, Seongnam, Korea.
- KMID: 2403472
- DOI: http://doi.org/10.4143/crt.2016.569
Abstract
- PURPOSE
This prospective study was conducted to determine the incidence of lacrimal drainage obstruction (LDO) during S-1 chemotherapy and evaluate the association between the development of LDO and the concentrations of ingredients/metabolites of S-1 in tears and plasma.
MATERIALS AND METHODS
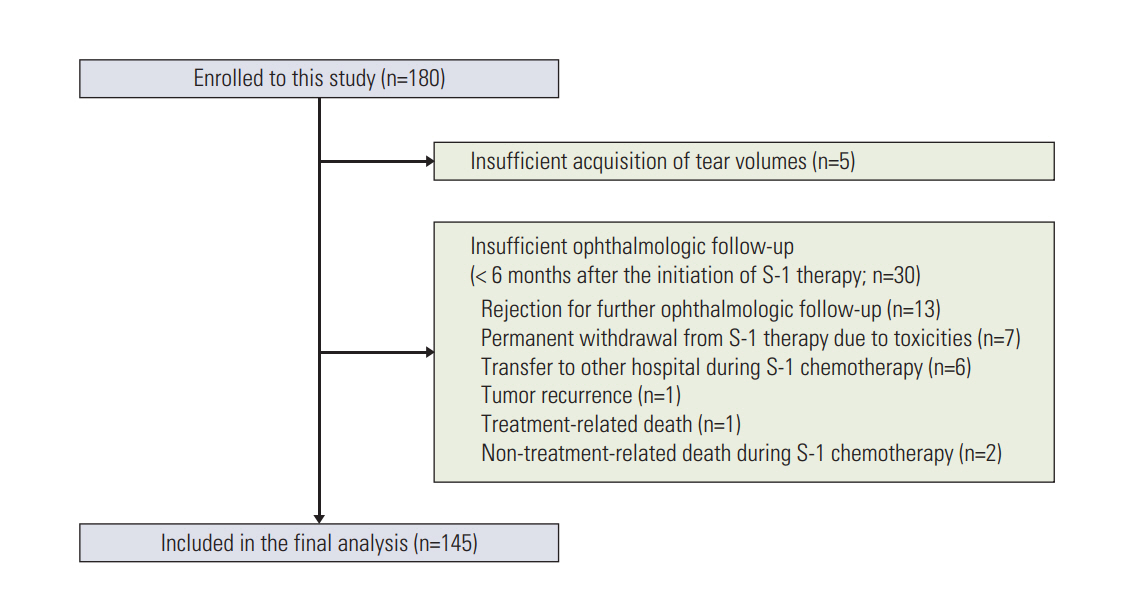
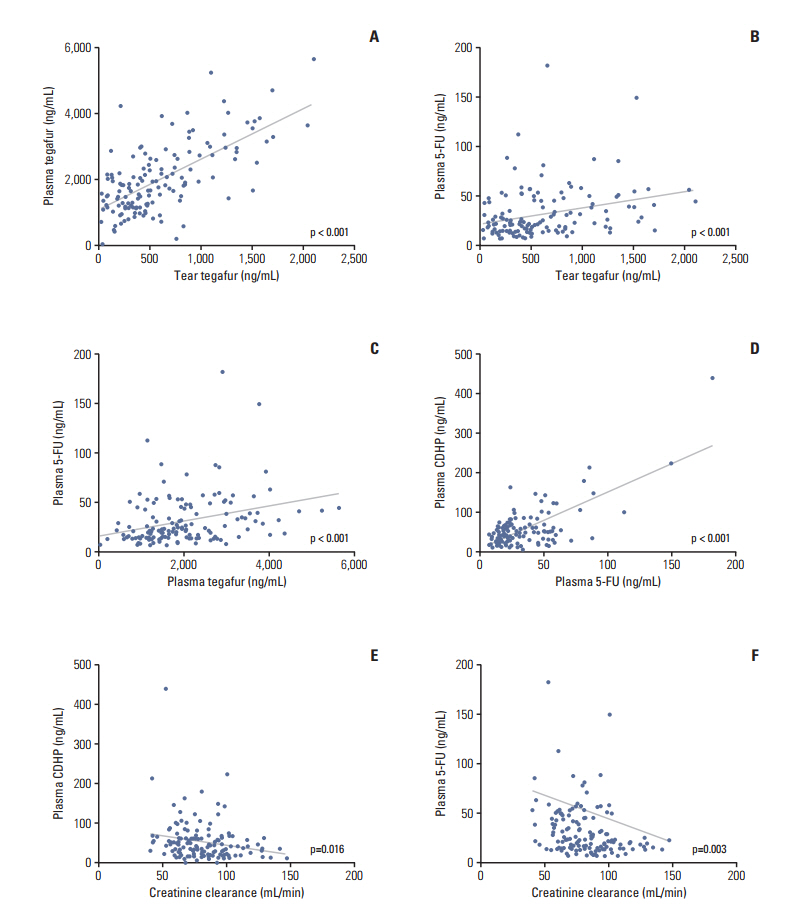
A total of 145 patients with gastric cancer who received adjuvant S-1 therapy were enrolled. Ophthalmologic examinations were performed regularly during S-1 chemotherapy. Concentrations of tegafur, 5-chloro-2,4-dihydroxypyridine (CDHP), and 5-fluorouracil at steady-state trough level were measured in both tears and plasma.
RESULTS
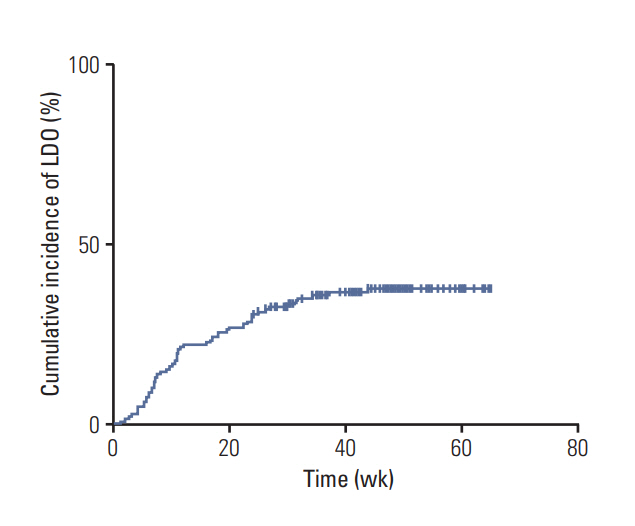
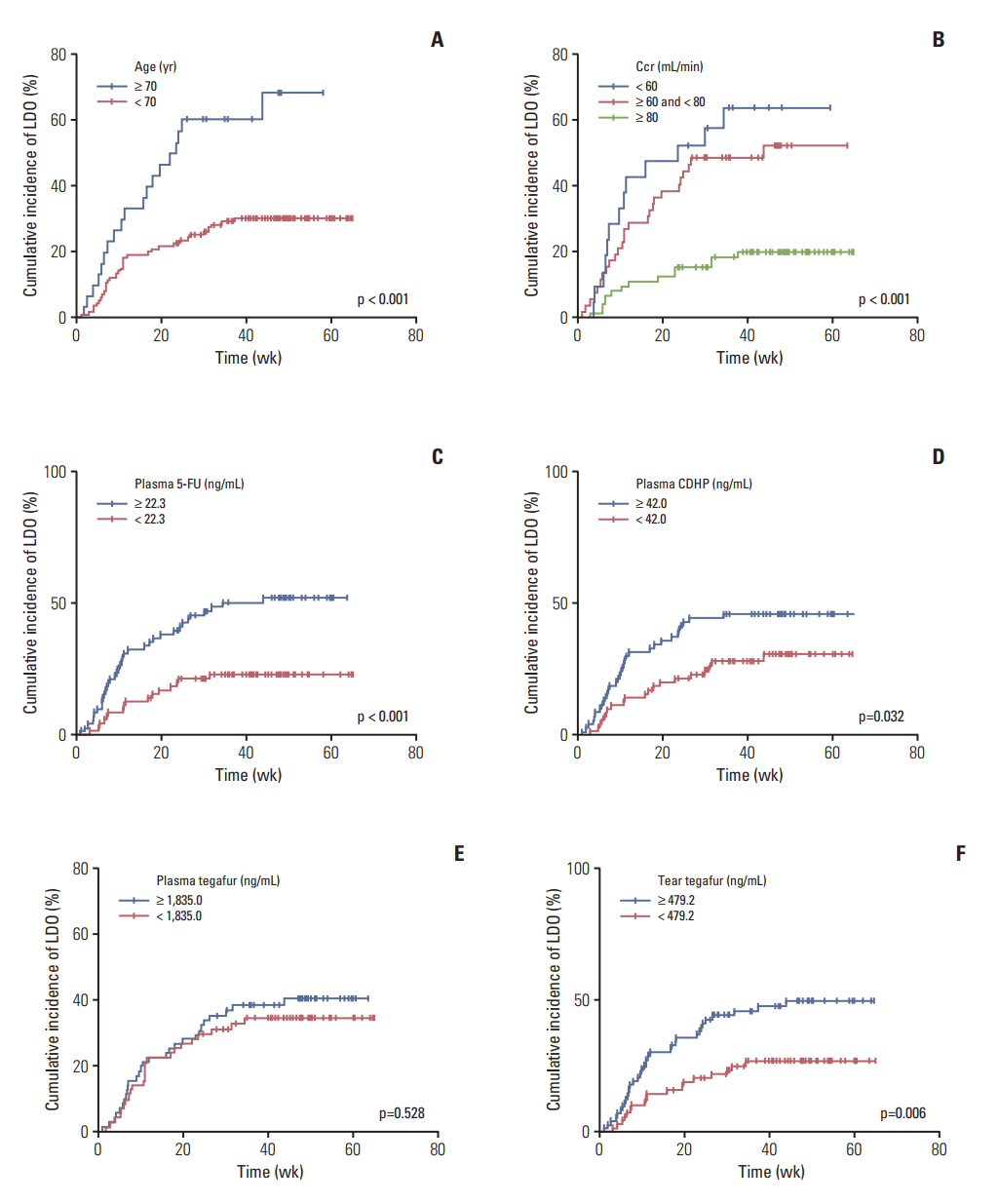
Fifty-three patients (37%) developed LDO. The median time to the onset of LDO was 10.9 weeks, and LDO developed most frequently in the nasolacrimal duct. Univariable analyses revealed that an older age (≥ 70 years), creatinine clearance rate (Ccr) < 80 mL/min, 5-fluorouracil concentration in plasma ≥ 22.3 ng/mL (median), CDHP concentration in plasma ≥ 42.0 ng/mL (median), and tegafur concentration in tears ≥ 479.2 ng/mL (median) were related to increased development of LDO. Multivariable analysis indicated that a high plasma 5-fluorouracil concentration was predictive of increased development of LDO (hazard ratio, 2.02; p=0.040), along with older age and decreased Ccr. Patients with LDO also developed S-1-related non-hematologic toxicity more frequently than those without LDO (p=0.016).
CONCLUSION
LDO is a frequent adverse event during S-1 chemotherapy. An older age, decreased Ccr, and high plasma 5-fluorouracil concentration were found to be independent risk factors for LDO. The high incidence of LDO warrants regular ophthalmologic examination and early intervention in patients receiving S-1 therapy.
MeSH Terms
Figure
Reference
-
References
1. Satoh T, Sakata Y. S-1 for the treatment of gastrointestinal cancer. Expert Opin Pharmacother. 2012; 13:1943–59.
Article2. Kim N, Park C, Park DJ, Kim HH, Kim S, Kim YJ, et al. Lacrimal drainage obstruction in gastric cancer patients receiving S-1 chemotherapy. Ann Oncol. 2012; 23:2065–71.
Article3. Kitamura H, Miyanaga T, Shin H, Fujita M, Miyazaki M, Yagi D, et al. Investigation of epiphora following S-1 therapy. Gan To Kagaku Ryoho. 2011; 38:259–62.4. Sasaki T, Miyashita H, Miyanaga T, Yamamoto K, Sugiyama K. Dacryoendoscopic observation and incidence of canalicular obstruction/stenosis associated with S-1, an oral anticancer drug. Jpn J Ophthalmol. 2012; 56:214–8.
Article5. Sato K, Nishimura S. Adverse effects of the oral anticancer drug s-1: lacrimal passage impairment and specific features of corneal epitheliopathy. Open Ophthalmol J. 2013; 7:85–6.
Article6. Kim SJ, Kim YJ, Kim JH, Park DJ, Kim HH, Lee JS, et al. Safety, compliance, and predictive parameters for dosage modification in adjuvant S-1 chemotherapy for gastric cancer. Cancer Sci. 2013; 104:116–23.
Article7. Kim JY, Shin E, Kim JW, Lee HS, Lee DW, Kim SH, et al. Impact of intratumoral expression levels of fluoropyrimidine-metabolizing enzymes on treatment outcomes of adjuvant S-1 therapy in gastric cancer. PLoS One. 2015; 10:e0120324.
Article8. Liu K, Zhong D, Zou H, Chen X. Determination of tegafur, 5-fluorouracil, gimeracil and oxonic acid in human plasma using liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal. 2010; 52:550–6.
Article9. Esmaeli B, Ahmadi MA, Rivera E, Valero V, Hutto T, Jackson DM, et al. Docetaxel secretion in tears: association with lacrimal drainage obstruction. Arch Ophthalmol. 2002; 120:1180–2.10. Christophidis N, Vajda FJ, Lucas I, Louis WJ. Ocular side effects with 5-fluorouracil. Aust N Z J Med. 1979; 9:143–4.
Article11. Loprinzi CL, Love RR, Garrity JA, Ames MM. Cyclophosphamide, methotrexate, and 5-fluorouracil (CMF)-induced ocular toxicity. Cancer Invest. 1990; 8:459–65.
Article12. Esmaeli B, Valero V, Ahmadi MA, Booser D. Canalicular stenosis secondary to docetaxel (taxotere): a newly recognized side effect. Ophthalmology. 2001; 108:994–5.13. Paulsen FP, Thale AB, Hallmann UJ, Schaudig U, Tillmann BN. The cavernous body of the human efferent tear ducts: function in tear outflow mechanism. Invest Ophthalmol Vis Sci. 2000; 41:965–70.14. Thale A, Paulsen F, Rochels R, Tillmann B. Functional anatomy of the human efferent tear ducts: a new theory of tear outflow mechanism. Graefes Arch Clin Exp Ophthalmol. 1998; 236:674–8.
Article