Cancer Res Treat.
2015 Oct;47(4):781-789. 10.4143/crt.2014.261.
Results of a Phase II Study to Evaluate the Efficacy of Docetaxel and Carboplatin in Metastatic Malignant Melanoma Patients Who Failed First-Line Therapy Containing Dacarbazine
- Affiliations
-
- 1Division of Medical Oncology, Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea. ssj338@yuhs.ac
- 2Department of Dermatology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2403397
- DOI: http://doi.org/10.4143/crt.2014.261
Abstract
- PURPOSE
There is no standard second-line regimen for malignant melanoma patients with disease progression after first-line chemotherapy, and platinum-alkylating agents combined with paclitaxel have shown modest efficacy.
MATERIALS AND METHODS
We conducted a phase II, open-label, single-arm study to test the efficacy of docetaxel combined with carboplatin for malignant melanoma patients who failed previous treatment with dacarbazine. Intravenous docetaxel (35 mg/m2 on days 1 and 8 of each cycle) and carboplatin (area under the curve 3 on days 1 and 8 of each cycle) was administered every 21 days. Primary end point was objective response rate (ORR).
RESULTS
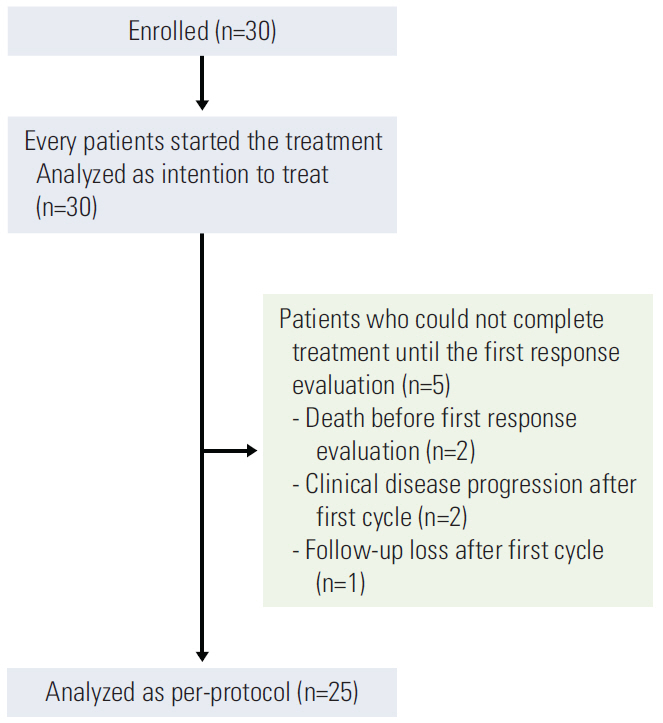
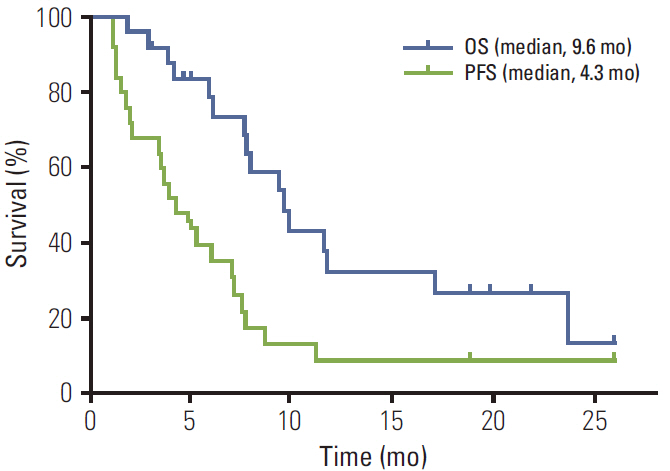
Thirty patients were enrolled in the study, and the median follow-up duration was 19.8 months. Among 25 per-protocol patients, there were three responders (1 with complete response and 2 with partial response) and 17 stable disease patients (ORR, 12.0%). Among the per-protocol population, the median progression-free survival (PFS) was 4.3 months and the median overall survival (OS) was 9.6 months. Uveal melanoma patients (n=9) showed the best prognosis compared to other subtypes (median PFS, 7.6 months; OS, 9.9 months). The most common grade 3 or 4 adverse event was neutropenia (n=15, 50.0%).
CONCLUSION
Docetaxel combined with carboplatin showed association with an acceptable safety profile and overall efficacy for patients with malignant melanoma who had progressed on chemotherapy containing dacarbazine.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014; 64:9–29.
Article2. Lee HY, Chay WY, Tang MB, Chio MT, Tan SH. Melanoma: differences between Asian and Caucasian patients. Ann Acad Med Singapore. 2012; 41:17–20.3. Ishihara K, Saida T, Otsuka F; Prognosis and Statistical Investigation Committee of the Japanese Skin Cancer Society. Statistical profiles of malignant melanoma and other skin cancers in Japan: 2007 update. Int J Clin Oncol. 2008; 13:33–41.
Article4. Comis RL. DTIC (NSC-45388) in malignant melanoma: a perspective. Cancer Treat Rep. 1976; 60:165–76.5. Atkins MB, Lotze MT, Dutcher JP, Fisher RI, Weiss G, Margolin K, et al. High-dose recombinant interleukin 2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999; 17:2105–16.
Article6. Rao RD, Holtan SG, Ingle JN, Croghan GA, Kottschade LA, Creagan ET, et al. Combination of paclitaxel and carboplatin as second-line therapy for patients with metastatic melanoma. Cancer. 2006; 106:375–82.
Article7. Flaherty KT, Lee SJ, Zhao F, Schuchter LM, Flaherty L, Kefford R, et al. Phase III trial of carboplatin and paclitaxel with or without sorafenib in metastatic melanoma. J Clin Oncol. 2013; 31:373–9.
Article8. Mhaidat NM, Wang Y, Kiejda KA, Zhang XD, Hersey P. Docetaxel-induced apoptosis in melanoma cells is dependent on activation of caspase-2. Mol Cancer Ther. 2007; 6:752–61.
Article9. Mhaidat NM, Zhang XD, Jiang CC, Hersey P. Docetaxel-induced apoptosis of human melanoma is mediated by activation of c-Jun NH2-terminal kinase and inhibited by the mitogen-activated protein kinase extracellular signal-regulated kinase 1/2 pathway. Clin Cancer Res. 2007; 13:1308–14.
Article10. Hotchkiss KA, Ashton AW, Mahmood R, Russell RG, Sparano JA, Schwartz EL. Inhibition of endothelial cell function in vitro and angiogenesis in vivo by docetaxel (Taxotere): association with impaired repositioning of the microtubule organizing center. Mol Cancer Ther. 2002; 1:1191–200.11. Grant DS, Williams TL, Zahaczewsky M, Dicker AP. Comparison of antiangiogenic activities using paclitaxel (taxol) and docetaxel (taxotere). Int J Cancer. 2003; 104:121–9.
Article12. Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, et al. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005; 353:2135–47.
Article13. Griewank KG, Westekemper H, Murali R, Mach M, Schilling B, Wiesner T, et al. Conjunctival melanomas harbor BRAF and NRAS mutations and copy number changes similar to cutaneous and mucosal melanomas. Clin Cancer Res. 2013; 19:3143–52.
Article14. Perrin C, Pracht M, Talour K, Adamski H, Cumin I, Porneuf M, et al. Metastatic melanoma: results of 'classical' second-line treatment with cytotoxic chemotherapies. J Dermatolog Treat. 2014; 25:396–400.
Article15. Eisen T, Trefzer U, Hamilton A, Hersey P, Millward M, Knight RD, et al. Results of a multicenter, randomized, double-blind phase 2/3 study of lenalidomide in the treatment of pretreated relapsed or refractory metastatic malignant melanoma. Cancer. 2010; 116:146–54.
Article16. Hauschild A, Agarwala SS, Trefzer U, Hogg D, Robert C, Hersey P, et al. Results of a phase III, randomized, placebocontrolled study of sorafenib in combination with carboplatin and paclitaxel as second-line treatment in patients with unresectable stage III or stage IV melanoma. J Clin Oncol. 2009; 27:2823–30.
Article17. Singh AD, Turell ME, Topham AK. Uveal melanoma: trends in incidence, treatment, and survival. Ophthalmology. 2011; 118:1881–5.
Article18. Diener-West M, Reynolds SM, Agugliaro DJ, Caldwell R, Cumming K, Earle JD, et al. Development of metastatic disease after enrollment in the COMS trials for treatment of choroidal melanoma: Collaborative Ocular Melanoma Study Group Report No. 26. Arch Ophthalmol. 2005; 123:1639–43.19. Bhatia S, Moon J, Margolin KA, Weber JS, Lao CD, Othus M, et al. Phase II trial of sorafenib in combination with carboplatin and paclitaxel in patients with metastatic uveal melanoma: SWOG S0512. PLoS One. 2012; 7:e48787.
Article20. Carvajal RD, Sosman JA, Quevedo JF, Milhem MM, Joshua AM, Kudchadkar RR, et al. Effect of selumetinib vs chemotherapy on progression-free survival in uveal melanoma: a randomized clinical trial. JAMA. 2014; 311:2397–405.21. Schmittel A, Scheulen ME, Bechrakis NE, Strumberg D, Baumgart J, Bornfeld N, et al. Phase II trial of cisplatin, gemcitabine and treosulfan in patients with metastatic uveal melanoma. Melanoma Res. 2005; 15:205–7.
Article22. Van Raamsdonk CD, Bezrookove V, Green G, Bauer J, Gaugler L, O'Brien JM, et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature. 2009; 457:599–602.
Article23. Van Raamsdonk CD, Griewank KG, Crosby MB, Garrido MC, Vemula S, Wiesner T, et al. Mutations in GNA11 in uveal melanoma. N Engl J Med. 2010; 363:2191–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- New Systemic Treatment for Malignant Melanoma
- A Case of Metastatic Malignant Melanoma of the Ovary
- Efficacy of administration of weekly docetaxel combined with platinum as a first-line treatment for patients with advanced non-small cell lung cancer
- Comparison of High Dose Interferon-α2b Immunotherapy and Dacarbazine Chemotherapy as Postoperative Treatment of Malignant Melanoma
- A Case of Metastatic Malignant Melanoma of the Liver Resulting from Choroidal Melanoma