Cancer Res Treat.
2015 Oct;47(4):727-737. 10.4143/crt.2014.018.
Dosimetric and Clinical Influence of 3D Versus 2D Planning in Postoperative Radiation Therapy for Gastric Cancer
- Affiliations
-
- 1Department of Radiation Oncology, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea.
- 2Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. ahnyc@skku.edu
- 3Department of Radiation Oncology, National Oncology Scientific Centre, Tashkent, Uzbekistan.
- KMID: 2403391
- DOI: http://doi.org/10.4143/crt.2014.018
Abstract
- PURPOSE
The purpose of this study is to investigate the dosimetric and clinical influence of computed tomography-based (3-dimensional [3D]) simulation versus conventional 2-dimensional (2D)-based simulation in postoperative chemoradiotherapy (CRT) for patients with advanced gastric cancer in terms of parallel opposed anteroposterior-posteroanterior field arrangement.
MATERIALS AND METHODS
A retrospective stage-matched cohort study was conducted in 158 patients treated with adjuvant CRT following curative surgery and D2 dissection from 2006 to 2008 at Samsung Medical Center: 98 patients in the 3D group; and 60 patients in the 2D group. For comparison of the dosimetric parameters between 3D plan and 2D plan, second sets of radiation treatment plans were generated according to the same target delineation method used in the 2D group for each patient in the 3D group (V2D). Acute toxicity, recurrence, and survival were analyzed. The median follow-up period was 28 months (range, 5 to 51 months).
RESULTS
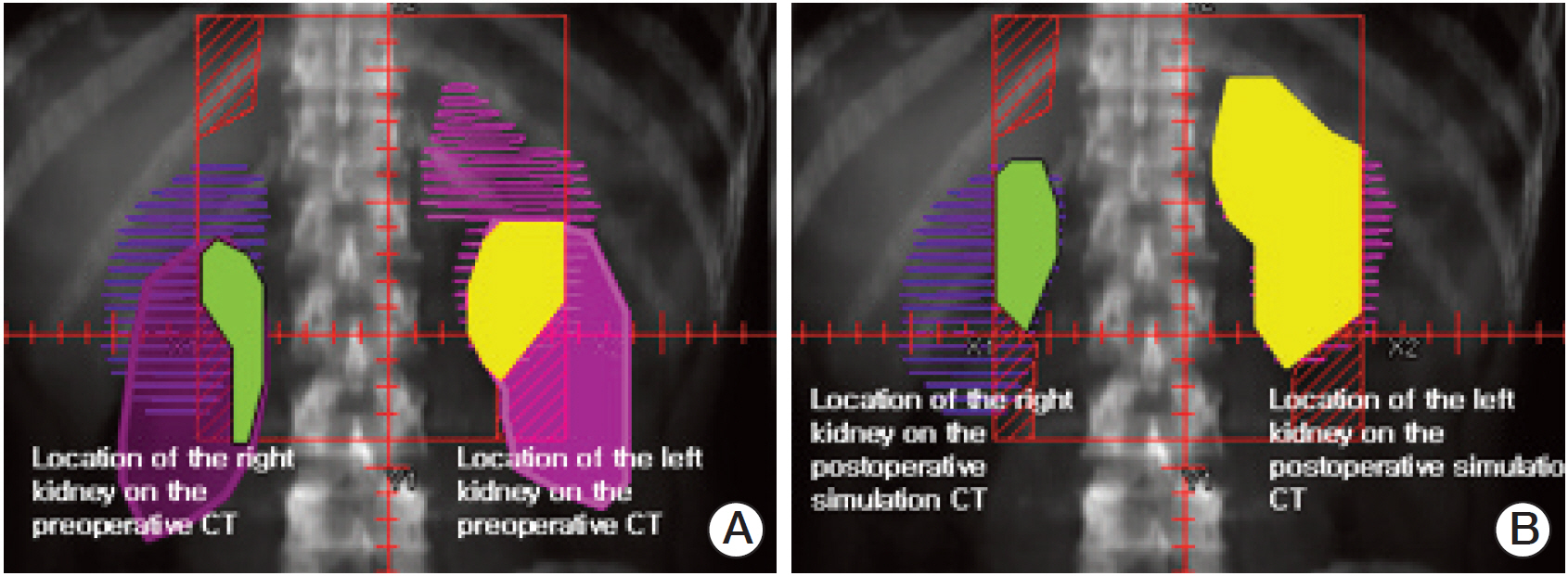
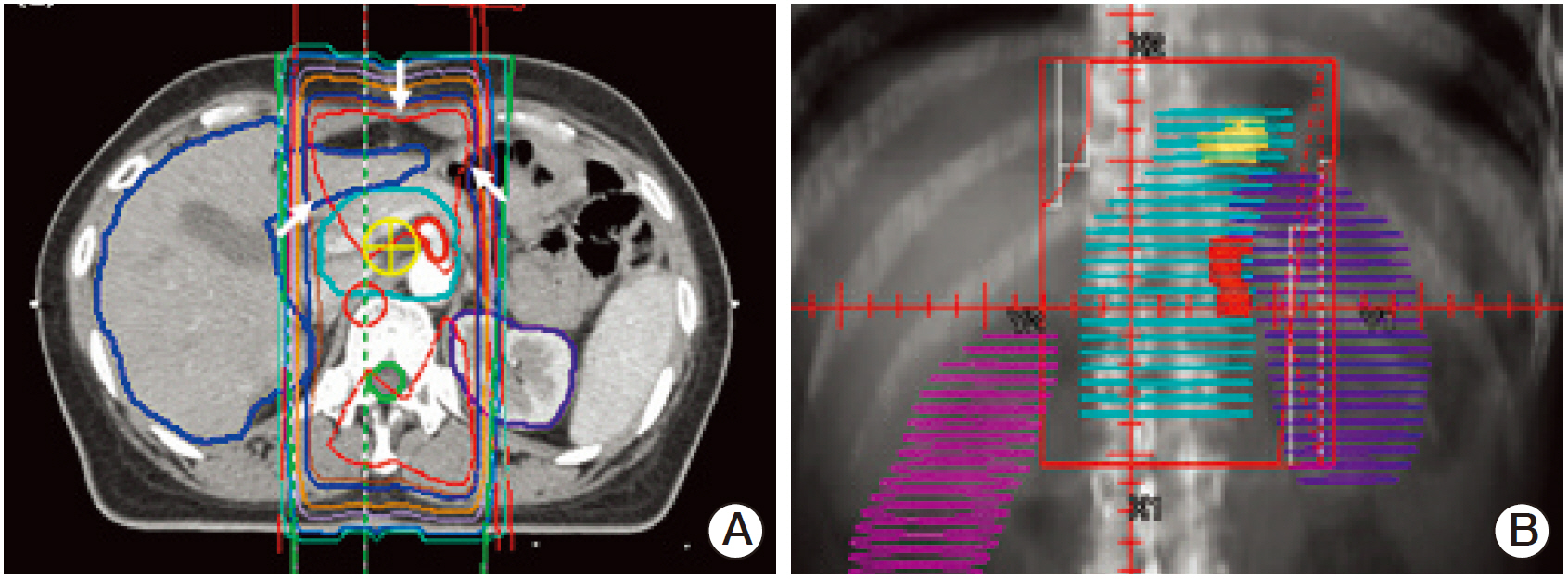
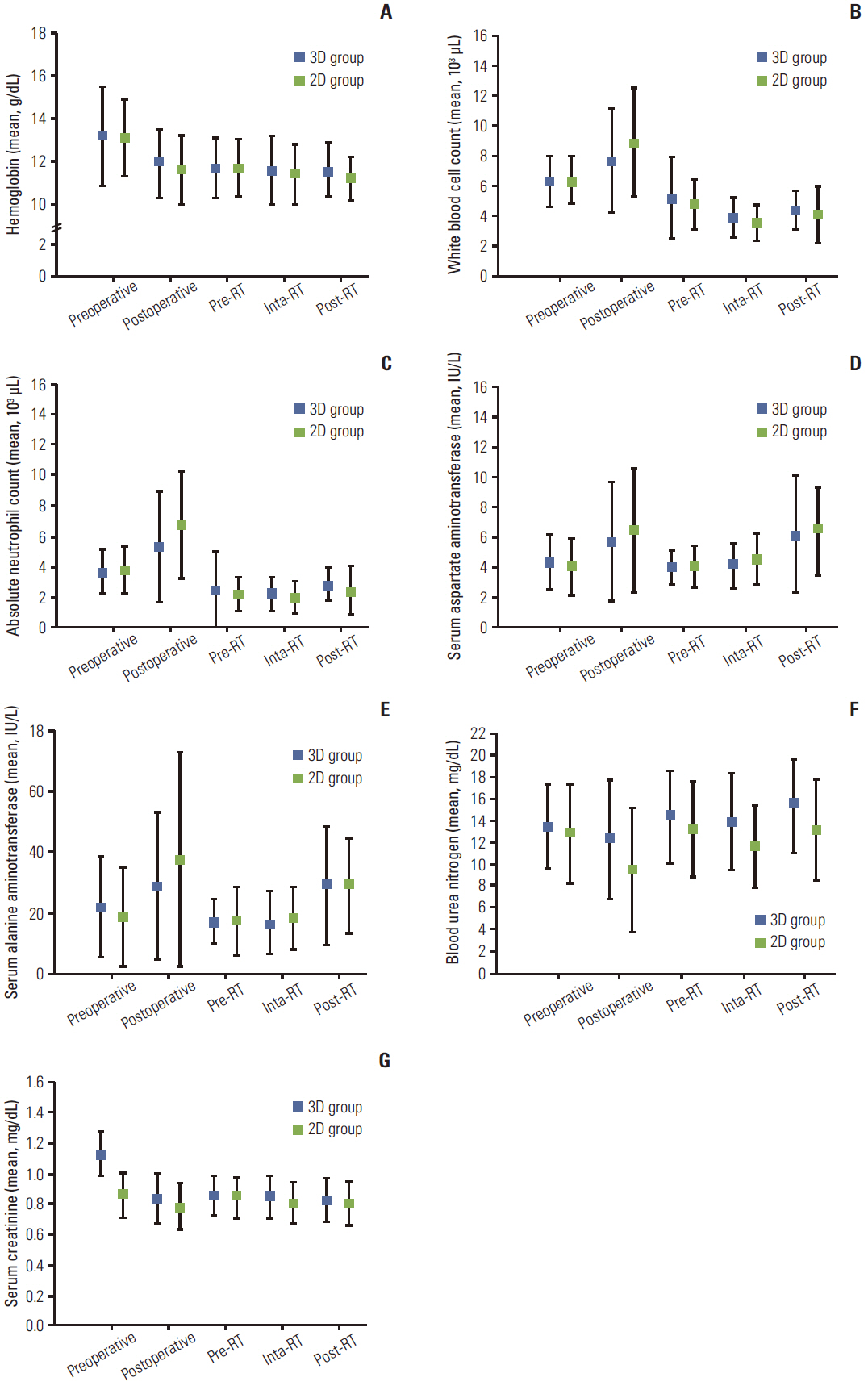
The 3D group showed better dose-volume histogram (DVH) profiles than the V2D group for all dosimetric parameters, including the kidneys, liver, spinal cord, duodenum, pancreas, and bowel. However, no difference in acute gastrointestinal toxicity and survival outcomes was observed between the 3D group and the 2D group.
CONCLUSION
The 3D plan enabled precise delineation of the target volume and organs at risk by visualization of geometric changes in the internal organs after surgery. The DVH of normal tissues in the 3D plan was superior to that of the V2D plan, but similar clinical features were observed between the 3D group and the 2D group.
MeSH Terms
Figure
Reference
-
References
1. Won YJ, Sung J, Jung KW, Kong HJ, Park S, Shin HR, et al. Nationwide cancer incidence in Korea, 2003-2005. Cancer Res Treat. 2009; 41:122–31.2. Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol. 2006; 24:2137–50.3. Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001; 345:725–30.4. Smalley SR, Gunderson L, Tepper J, Martenson JA Jr, Minsky B, Willett C, et al. Gastric surgical adjuvant radiotherapy consensus report: rationale and treatment implementation. Int J Radiat Oncol Biol Phys. 2002; 52:283–93.5. Tepper JE, Gunderson LL. Radiation treatment parameters in the adjuvant postoperative therapy of gastric cancer. Semin Radiat Oncol. 2002; 12:187–95.6. Lim DH, Kim DY, Kang MK, Kim YI, Kang WK, Park CK, et al. Patterns of failure in gastric carcinoma after D2 gastrectomy and chemoradiotherapy: a radiation oncologist's view. Br J Cancer. 2004; 91:11–7.7. Nam H, Lim DH, Kim S, Kang WK, Sohn TS, Noh JH, et al. A new suggestion for the radiation target volume after a subtotal gastrectomy in patients with stomach cancer. Int J Radiat Oncol Biol Phys. 2008; 71:448–55.8. Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma: 2nd English edition. Gastric Cancer. 1998; 1:10–24.9. Leong T, Willis D, Joon DL, Condron S, Hui A, Ngan SY. 3D conformal radiotherapy for gastric cancer: results of a comparative planning study. Radiother Oncol. 2005; 74:301–6.10. Soyfer V, Corn BW, Melamud A, Alani S, Tempelhof H, Agai R, et al. Three-dimensional non-coplanar conformal radiotherapy yields better results than traditional beam arrangements for adjuvant treatment of gastric cancer. Int J Radiat Oncol Biol Phys. 2007; 69:364–9.11. Alani S, Soyfer V, Strauss N, Schifter D, Corn BW. Limited advantages of intensity-modulated radiotherapy over 3D conformal radiation therapy in the adjuvant management of gastric cancer. Int J Radiat Oncol Biol Phys. 2009; 74:562–6.12. Emami B, Lyman J, Brown A, Coia L, Goitein M, Munzenrider JE, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys. 1991; 21:109–22.13. Peschel RE, Chen M, Seashore J. The treatment of massive hepatomegaly in stage IV-S neuroblastoma. Int J Radiat Oncol Biol Phys. 1981; 7:549–53.14. Thompson PL, Mackay IR, Robson GS, Wall AJ. Late radiation nephritis after gastric x-irradiation for peptic ulcer. Q J Med. 1971; 40:145–57.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Who Really Benefits from 3D-Based Planning of Brachytherapy for Cervical Cancer?
- Difference in the Set-up Margin between 2D Conventional and 3D CT Based Planning in Patients with Early Breast Cancer
- Radiation therapy for gastric mucosa-associated lymphoid tissue lymphoma: dose-volumetric analysis and its clinical implications
- Dosimetric comparison of axilla and groin radiotherapy techniques for high-risk and locally advanced skin cancer
- Correlation of Conventional and Conformal Plan Parameters for Predicting Radiation Pneumonitis in Patients Treated with Breast Cancer