Allergy Asthma Immunol Res.
2018 Mar;10(2):121-130. 10.4168/aair.2018.10.2.121.
Therapeutic Effect of Omalizumab in Severe Asthma: A Real-World Study in Korea
- Affiliations
-
- 1Department of Allergy and Clinical Immunology, Ajou University School of Medicine, Suwon, Korea. hspark@ajou.ac.kr
- 2Department of Statistics, Clinical Trial Center, Ajou University Medical Center, Suwon, Korea.
- KMID: 2402971
- DOI: http://doi.org/10.4168/aair.2018.10.2.121
Abstract
- PURPOSE
Omalizumab, an anti-immunoglobulin E (IgE) monoclonal antibody, has proved to be effective for the treatment of severe asthma. However, there is no direct evidence of effectiveness of omalizumab in Korean patients with severe asthma. We sought to evaluate the real-world effectiveness of omalizumab in Korean adult patients suffering from severe asthma and to identify predictors of favorable response.
METHODS
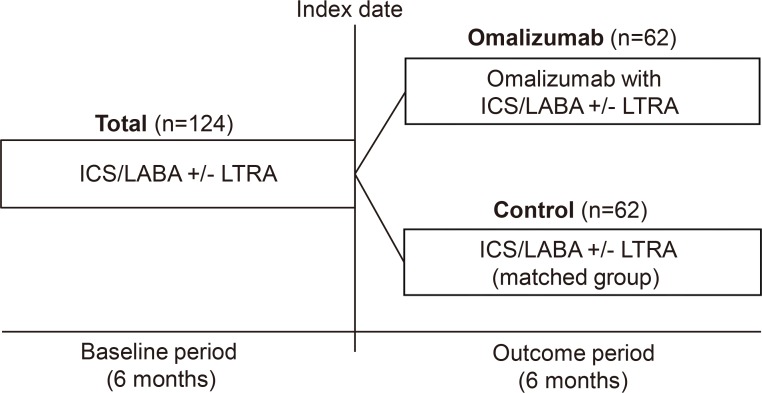
A retrospective analysis of electrical medical records was performed on severe allergic asthmatic patients with omalizumab treatment group (OT group) for more than 6 months between March 2008 and February 2016. Propensity score matching was applied to define the standardized treatment control group (STC group) treated without omalizumab. Asthma-related outcomes were compared between the 2 groups, and analyzed before and after omalizumab use in the OT group. Responders to treatment were defined as patients showing >50% reduction in asthma exacerbations and/or systemic steroid requirement during the outcome period.
RESULTS
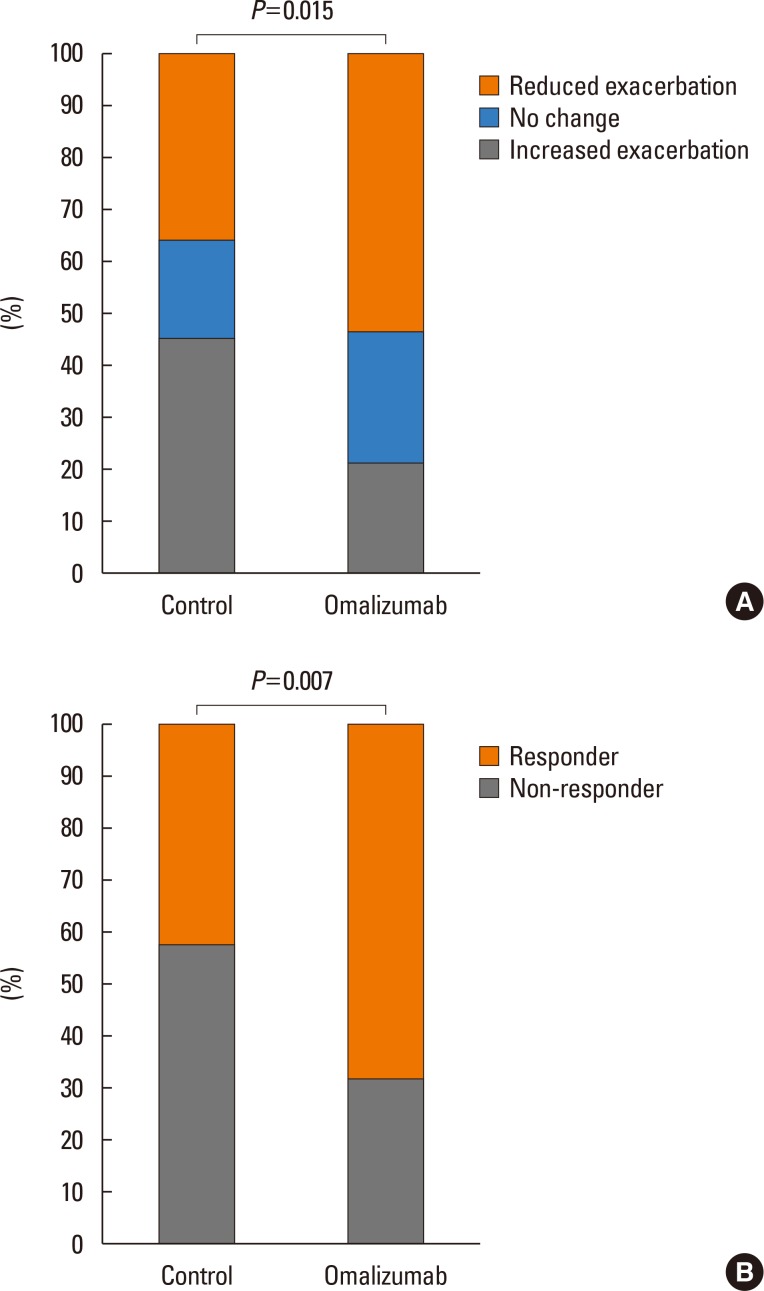
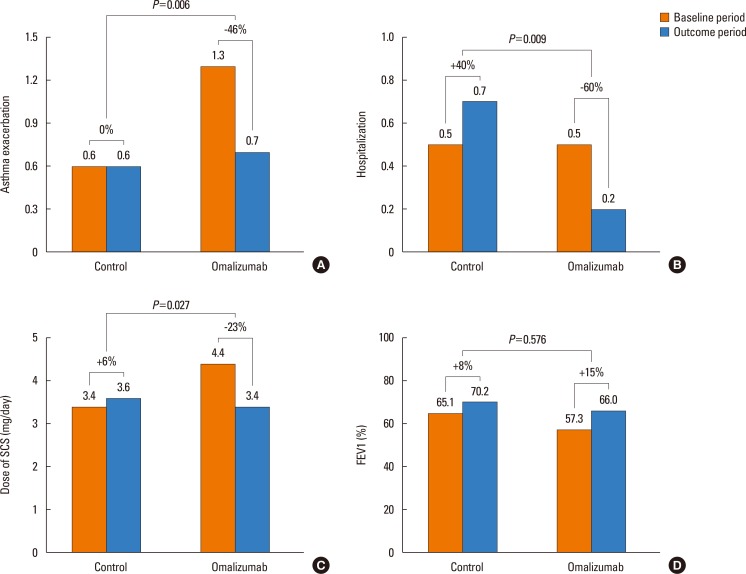
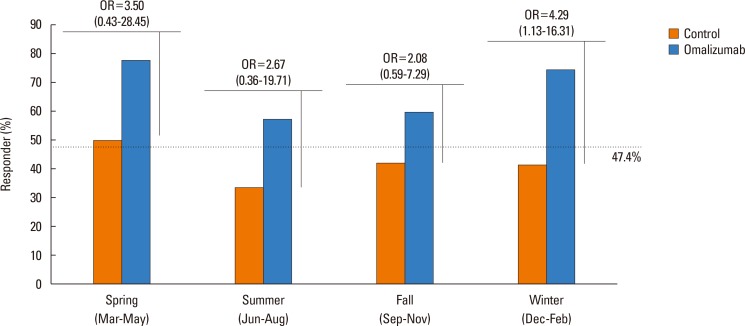
One hundred twenty-four patients with severe asthma (62 in the OT group; 62 in the STC group) were enrolled in the study. Proportion of patients having the reduction of asthma exacerbation (53.2% vs 35.5%, P=0.015) and the rate of responders (67.7% vs 41.9%, P=0.007) were significantly higher in the OT group than in the STC group. Significant reductions were noted in asthma exacerbation (P=0.006), hospitalization (P=0.009), hospitalization days (P=0.006), systemic corticosteroid requirements (P=0.027), and sputum eosinophil count (P=0.031) in OT group compared with STC group. There were no significant differences in changes of forced expiratory volume in the 1 second (FEV1) levels between the 2 groups. No predictors of responders were found for omalizumab treatment.
CONCLUSIONS
Omalizumab can reduce exacerbations/hospitalization/systemic steroid burst in Korean adult patients with severe asthma.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Update on the Management of Nonsteroidal Anti-Inflammatory Drug Hypersensitivity
Wan Yin Winnie Yeung, Hae Sim Park
Yonsei Med J. 2020;61(1):4-14. doi: 10.3349/ymj.2020.61.1.4.Is Omalizumab a Problem-Solving Remedy in Severe Asthma?
Doh Hyung Kim, Young-Koo Jee
Allergy Asthma Immunol Res. 2018;10(2):95-96. doi: 10.4168/aair.2018.10.2.95.
Reference
-
1. Chung KF, Wenzel SE, Brozek JL, Bush A, Castro M, Sterk PJ, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014; 43:343–373. PMID: 24337046.2. Sullivan PW, Slejko JF, Ghushchyan VH, Sucher B, Globe DR, Lin SL, et al. The relationship between asthma, asthma control and economic outcomes in the United States. J Asthma. 2014; 51:769–778. PMID: 24697738.
Article3. Zeiger RS, Schatz M, Dalal AA, Qian L, Chen W, Ngor EW, et al. Utilization and costs of severe uncontrolled asthma in a managed-care setting. J Allergy Clin Immunol Pract. 2016; 4:120–129.e3. PMID: 26439182.
Article4. Platts-Mills TA. The role of immunoglobulin E in allergy and asthma. Am J Respir Crit Care Med. 2001; 164:S1–S5. PMID: 11704610.
Article5. Schulman ES. Development of a monoclonal anti-immunoglobulin E antibody (omalizumab) for the treatment of allergic respiratory disorders. Am J Respir Crit Care Med. 2001; 164:S6–S11. PMID: 11704611.
Article6. Pereira Santos MC, Campos Melo A, Caetano A, Caiado J, Mendes A, Pereira Barbosa M, et al. Longitudinal study of the expression of FcεRI and IgE on basophils and dendritic cells in association with basophil function in two patients with severe allergic asthma treated with omalizumab. Eur Ann Allergy Clin Immunol. 2015; 47:38–40. PMID: 25781192.7. Niven RM, Saralaya D, Chaudhuri R, Masoli M, Clifton I, Mansur AH, et al. Impact of omalizumab on treatment of severe allergic asthma in UK clinical practice: a UK multicentre observational study (the APEX II study). BMJ Open. 2016; 6:e011857.
Article8. Bousquet J, Cabrera P, Berkman N, Buhl R, Holgate S, Wenzel S, et al. The effect of treatment with omalizumab, an anti-IgE antibody, on asthma exacerbations and emergency medical visits in patients with severe persistent asthma. Allergy. 2005; 60:302–308. PMID: 15679714.
Article9. Sattler C, Garcia G, Humbert M. Novel targets of omalizumab in asthma. Curr Opin Pulm Med. 2017; 23:56–61. PMID: 27798419.
Article10. Teach SJ, Gill MA, Togias A, Sorkness CA, Arbes SJ Jr, Calatroni A, et al. Preseasonal treatment with either omalizumab or an inhaled corticosteroid boost to prevent fall asthma exacerbations. J Allergy Clin Immunol. 2015; 136:1476–1485. PMID: 26518090.
Article11. Hayashi H, Mitsui C, Nakatani E, Fukutomi Y, Kajiwara K, Watai K, et al. Omalizumab reduces cysteinyl leukotriene and 9α,11β-prostaglandin F2 overproduction in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. 2016; 137:1585–1587.e4. PMID: 26559322.12. Price D, Brusselle G, Roche N, Freeman D, Chisholm A. Real-world research and its importance in respiratory medicine. Breathe (Sheff). 2015; 11:26–38. PMID: 26306101.
Article13. Norman G, Faria R, Paton F, Llewellyn A, Fox D, Palmer S, et al. Omalizumab for the treatment of severe persistent allergic asthma: a systematic review and economic evaluation. Health Technol Assess. 2013; 17:1–342.
Article14. Nair P, Wenzel S, Rabe KF, Bourdin A, Lugogo NL, Kuna P, et al. Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med. 2017; 376:2448–2458. PMID: 28530840.
Article15. Nguyen VQ, Ulrik CS. Measures to reduce maintenance therapy with oral corticosteroid in adults with severe asthma. Allergy Asthma Proc. 2016; 37:125–139. PMID: 27931289.
Article16. Barnes N, Menzies-Gow A, Mansur AH, Spencer D, Percival F, Radwan A, et al. Effectiveness of omalizumab in severe allergic asthma: a retrospective UK real-world study. J Asthma. 2013; 50:529–536. PMID: 23574000.
Article17. Costello RW, Long DA, Gaine S, Mc Donnell T, Gilmartin JJ, Lane SJ. Therapy with omalizumab for patients with severe allergic asthma improves asthma control and reduces overall healthcare costs. Ir J Med Sci. 2011; 180:637–641. PMID: 21557095.
Article18. Brusselle G, Michils A, Louis R, Dupont L, Van de Maele B, Delobbe A, et al. “Real-life” effectiveness of omalizumab in patients with severe persistent allergic asthma: the PERSIST study. Respir Med. 2009; 103:1633–1642. PMID: 19619998.
Article19. Tzortzaki EG, Georgiou A, Kampas D, Lemessios M, Markatos M, Adamidi T, et al. Long-term omalizumab treatment in severe allergic asthma: the South-Eastern Mediterranean “real-life” experience. Pulm Pharmacol Ther. 2012; 25:77–82. PMID: 22155001.
Article20. Cazzola M, Camiciottoli G, Bonavia M, Gulotta C, Ravazzi A, Alessandrini A, et al. Italian real-life experience of omalizumab. Respir Med. 2010; 104:1410–1416. PMID: 20483574.
Article21. Molimard M, de Blay F, Didier A, Le Gros V. Effectiveness of omalizumab (Xolair) in the first patients treated in real-life practice in France. Respir Med. 2008; 102:71–76. PMID: 17920257.
Article22. Molimard M, Buhl R, Niven R, Le Gros V, Thielen A, Thirlwell J, et al. Omalizumab reduces oral corticosteroid use in patients with severe allergic asthma: real-life data. Respir Med. 2010; 104:1381–1385. PMID: 20599369.
Article23. Braunstahl GJ, Chen CW, Maykut R, Georgiou P, Peachey G, Bruce J. The eXpeRience registry: the ‘real-world’ effectiveness of omalizumab in allergic asthma. Respir Med. 2013; 107:1141–1151. PMID: 23721684.
Article24. Kupryś-Lipińska I, Molińska K, Kuna P. The effect of omalizumab on eosinophilic inflammation of the respiratory tract in patients with allergic asthma. Pneumonol Alergol Pol. 2016; 84:232–243. PMID: 27435350.25. Li J, Kang J, Wang C, Yang J, Wang L, Kottakis I, et al. Omalizumab improves quality of life and asthma control in Chinese patients with moderate to severe asthma: a Randomized Phase III Study. Allergy Asthma Immunol Res. 2016; 8:319–328. PMID: 27126725.
Article26. Fajt ML, Wenzel SE. Development of new therapies for severe asthma. Allergy Asthma Immunol Res. 2017; 9:3–14. PMID: 27826957.
Article27. Carr TF, Berdnikovs S, Simon HU, Bochner BS, Rosenwasser LJ. Eosinophilic bioactivities in severe asthma. World Allergy Organ J. 2016; 9:21. PMID: 27386041.
Article28. Bousquet J, Rabe K, Humbert M, Chung KF, Berger W, Fox H, et al. Predicting and evaluating response to omalizumab in patients with severe allergic asthma. Respir Med. 2007; 101:1483–1492. PMID: 17339107.
Article29. Hanania NA, Wenzel S, Rosén K, Hsieh HJ, Mosesova S, Choy DF, et al. Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med. 2013; 187:804–811. PMID: 23471469.30. Bousquet J, Wenzel S, Holgate S, Lumry W, Freeman P, Fox H. Predicting response to omalizumab, an anti-IgE antibody, in patients with allergic asthma. Chest. 2004; 125:1378–1386. PMID: 15078749.
Article31. de Llano LP, Vennera MC, Álvarez FJ, Medina JF, Borderías L, Pellicer C, et al. Effects of omalizumab in non-atopic asthma: results from a Spanish multicenter registry. J Asthma. 2013; 50:296–301. PMID: 23350994.
Article32. Garcia G, Magnan A, Chiron R, Contin-Bordes C, Berger P, Taillé C, et al. A proof-of-concept, randomized, controlled trial of omalizumab in patients with severe, difficult-to-control, nonatopic asthma. Chest. 2013; 144:411–419. PMID: 23579324.
Article33. Pillai P, Chan YC, Wu SY, Ohm-Laursen L, Thomas C, Durham SR, et al. Omalizumab reduces bronchial mucosal IgE and improves lung function in non-atopic asthma. Eur Respir J. 2016; 48:1593–1601. PMID: 27824606.
Article34. Song WJ, Chang YS, Lim MK, Yun EH, Kim SH, Kang HR, et al. Staphylococcal enterotoxin sensitization in a community-based population: a potential role in adult-onset asthma. Clin Exp Allergy. 2014; 44:553–562. PMID: 24444125.
Article35. Smith-Norowitz TA, Mandal M, Joks R, Norowitz LT, Weaver D, Durkin HG, et al. IgE anti-respiratory syncytial virus antibodies detected in serum of pediatric patients with asthma. Hum Immunol. 2015; 76:519–524. PMID: 26074414.
Article36. Gonzalez-Barcala FJ, Aboal J, Valdes L, Carreira JM, Alvarez-Dobaño JM, Puga A, et al. Trends in adult asthma hospitalization: gender-age effect. Multidiscip Respir Med. 2011; 6:82–86. PMID: 22958944.
Article37. Won YK, Hwang TH, Roh EJ, Chung EH. Seasonal patterns of asthma in children and adolescents presenting at emergency departments in Korea. Allergy Asthma Immunol Res. 2016; 8:223–229. PMID: 26922932.
Article38. Altzibar JM, Tamayo-Uria I, De Castro V, Aginagalde X, Albizu MV, Lertxundi A, et al. Epidemiology of asthma exacerbations and their relation with environmental factors in the Basque Country. Clin Exp Allergy. 2015; 45:1099–1108. PMID: 25258133.
Article39. Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, et al. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011; 364:1005–1015. PMID: 21410369.
Article40. Kloepfer KM, Gern JE. Virus/allergen interactions and exacerbations of asthma. Immunol Allergy Clin North Am. 2010; 30:553–563. viiPMID: 21029938.
Article41. Buchheit KM, Laidlaw TM. Update on the management of aspirin-exacerbated respiratory disease. Allergy Asthma Immunol Res. 2016; 8:298–304. PMID: 27126722.
Article42. Bochenek G, Kuschill-Dziurda J, Szafraniec K, Plutecka H, Szczeklik A, Nizankowska-Mogilnicka E. Certain subphenotypes of aspirin-exacerbated respiratory disease distinguished by latent class analysis. J Allergy Clin Immunol. 2014; 133:98–103. 103.e1–103.e6. PMID: 23993879.
Article43. Johns CB, Laidlaw TM. Elevated total serum IgE in nonatopic patients with aspirin-exacerbated respiratory disease. Am J Rhinol Allergy. 2014; 28:287–289. PMID: 25197914.
Article44. Phillips-Angles E, Barranco P, Lluch-Bernal M, Dominguez-Ortega J, López-Carrasco V, Quirce S. Aspirin tolerance in patients with nonsteroidal anti-inflammatory drug-exacerbated respiratory disease following treatment with omalizumab. J Allergy Clin Immunol Pract. 2017; 5:842–845. PMID: 28117272.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Is Omalizumab a Problem-Solving Remedy in Severe Asthma?
- Omalizumab and unmet needs in severe asthma and allergic comorbidities in Japanese children
- Real-life Efficacy of Omalizumab After 9 Years of Follow-up
- Omalizumab on Chronic Spontaneous Urticaria and Chronic Inducible Urticaria: A Real-World Study of Efficacy and Predictors of Treatment Outcome
- Omalizumab in recurring larynx angioedema: a case report