J Korean Med Sci.
2022 Jul;37(27):e211. 10.3346/jkms.2022.37.e211.
Omalizumab on Chronic Spontaneous Urticaria and Chronic Inducible Urticaria: A Real-World Study of Efficacy and Predictors of Treatment Outcome
- Affiliations
-
- 1Department of Dermatology, Hallym University Sacred Heart Hospital, Anyang, Korea
- 2Department of Dermatology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2531249
- DOI: http://doi.org/10.3346/jkms.2022.37.e211
Abstract
- Background
Omalizumab is a very important drug for the treatment of chronic urticaria. Although omalizumab’s therapeutic efficacy has been demonstrated, data on real-world experiences in Korea, especially regarding chronic inducible urticaria (CIndU), are limited. This study attempted to compare the efficacy of omalizumab in Korean chronic spontaneous urticaria (CSU) and CIndU patients.
Methods
Fifty-two CSU and 29 CIndU patients were included and Urticaria Activity Score 7 (UAS7) at baseline, week 4, and week 12 was assessed retrospectively.
Results
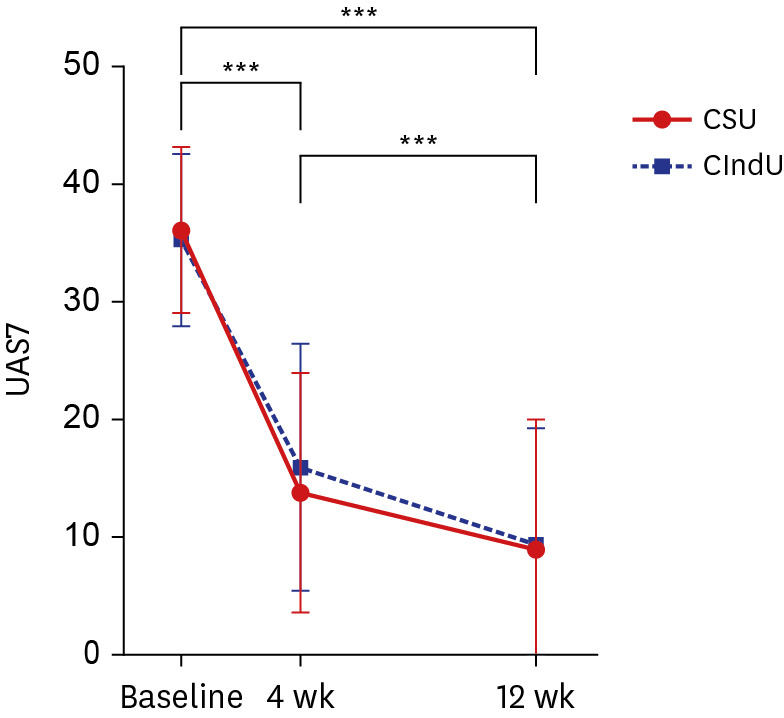
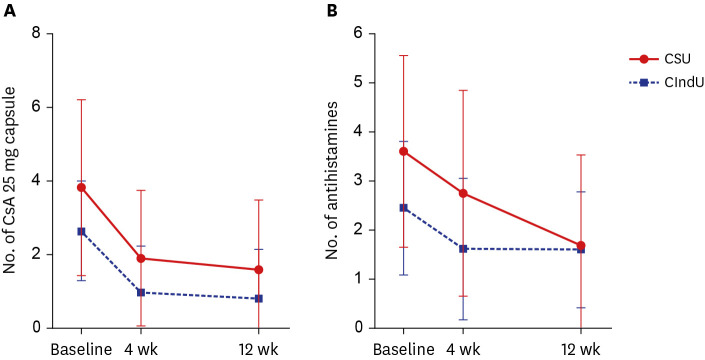
Omalizumab 150 mg significantly decreased UAS7 in both patients with CSU and CIndU with only one dose (P < 0.001). The significant decrease in the UAS7 scores of both groups of patients continued from weeks 4 to 12. Although there was no significant difference in treatment efficacy between the two groups, the symptoms of patients with CSU tended to improve faster; furthermore, the number of antihistamines administered daily reduced more significantly in this patient group (P = 0.047). Additionally, the decrease in the UAS7 score between baseline and week 12 and the response rate were higher in patients with CSU.
Conclusion
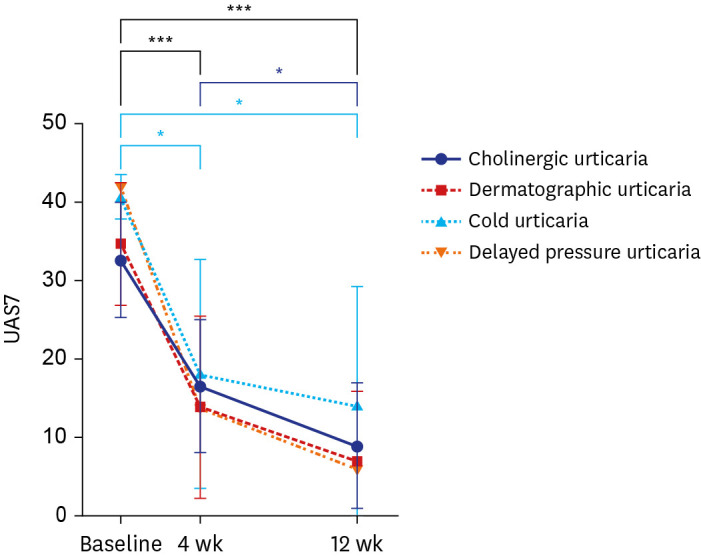
Omalizumab may be slightly more effective against CSU than against CIndU. Regarding the CIndU subtypes, dermatographic urticaria was associated with the greatest reduction in the UAS7 score, and patients with this condition showed the highest response rate, indicating the best effect of omalizumab. The duration of chronic urticaria was greater in non-responders than in responders (P = 0.025). Conversely, baseline immunoglobulin E levels were significantly higher in responders (P = 0.039).
Figure
Reference
-
1. Tharp MD, Bernstein JA, Kavati A, Ortiz B, MacDonald K, Denhaerynck K, et al. Benefits and harms of omalizumab treatment in adolescent and adult patients with chronic idiopathic (spontaneous) urticaria: a meta-analysis of “Real-world” evidence. JAMA Dermatol. 2019; 155(1):29–38. PMID: 30427977.
Article2. Nam YH, Kim JH, Jin HJ, Hwang EK, Shin YS, Ye YM, et al. Effects of omalizumab treatment in patients with refractory chronic urticaria. Allergy Asthma Immunol Res. 2012; 4(6):357–361. PMID: 23115733.
Article3. Lefévre AC, Deleuran M, Vestergaard C. A long term case series study of the effect of omalizumab on chronic spontaneous urticaria. Ann Dermatol. 2013; 25(2):242–245. PMID: 23717021.
Article4. Yu M, Terhorst-Molawi D, Altrichter S, Hawro T, Chen YD, Liu B, et al. Omalizumab in chronic inducible urticaria: a real-life study of efficacy, safety, predictors of treatment outcome and time to response. Clin Exp Allergy. 2021; 51(5):730–734. PMID: 33522024.
Article5. Exposito-Serrano V, Curto-Barredo L, Aguilera Peiro P, Gómez Armayones S, Serra-Baldrich E, Spertino J, et al. Omalizumab for the treatment of chronic inducible urticaria in 80 patients. Br J Dermatol. 2021; 184(1):167–168. PMID: 32730636.
Article6. Maurer M, Metz M, Brehler R, Hillen U, Jakob T, Mahler V, et al. Omalizumab treatment in patients with chronic inducible urticaria: a systematic review of published evidence. J Allergy Clin Immunol. 2018; 141(2):638–649. PMID: 28751232.
Article7. Kim JH, Park HS, Ye YM, Shin YS, Kang HR, Chung SJ, et al. Omalizumab treatment in patients with cholinergic urticaria: a real-world retrospective study in Korea. Allergy Asthma Immunol Res. 2020; 12(5):894–896. PMID: 32638568.
Article8. Kocatürk E, Can PK, Akbas PE, Copur M, Degirmentepe EN, Kızıltac K, et al. Management of chronic inducible urticaria according to the guidelines: a prospective controlled study. J Dermatol Sci. 2017; 87(1):60–69. PMID: 28314658.
Article9. Chen Y, Yu M, Huang X, Tu P, Shi P, Maurer M, et al. Omalizumab treatment and outcomes in Chinese patients with chronic spontaneous urticaria, chronic inducible urticaria, or both. World Allergy Organ J. 2021; 14(1):100501. PMID: 33510832.
Article10. Urgert MC, van den Elzen MT, Knulst AC, Fedorowicz Z, van Zuuren EJ. Omalizumab in patients with chronic spontaneous urticaria: a systematic review and GRADE assessment. Br J Dermatol. 2015; 173(2):404–415. PMID: 25891046.
Article11. Maurer M, Schütz A, Weller K, Schoepke N, Peveling-Oberhag A, Staubach P, et al. Omalizumab is effective in symptomatic dermographism-results of a randomized placebo-controlled trial. J Allergy Clin Immunol. 2017; 140(3):870–873.e5. PMID: 28389391.
Article12. Metz M, Ohanyan T, Church MK, Maurer M. Omalizumab is an effective and rapidly acting therapy in difficult-to-treat chronic urticaria: a retrospective clinical analysis. J Dermatol Sci. 2014; 73(1):57–62. PMID: 24060603.
Article13. Fialek M, Dezoteux F, Le Moing A, Karimova E, Ramdane N, Pape E, et al. Omalizumab in chronic inducible urticaria: a retrospective, real-life study. Ann Dermatol Venereol. 2021; 148(4):262–265. PMID: 34247853.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Treatment with biological products for chronic urticaria
- Emerging Therapies in Chronic Spontaneous Urticaria
- Omalizumab for Cyclosporine-resistant Chronic Spontaneous Urticaria: A Retrospective Study of a Single Center
- Analysis of Predictive Factors of Response to Omalizumab Treatment in Chronic Spontaneous Urticaria Patients
- A Long Term Case Series Study of the Effect of Omalizumab on Chronic Spontaneous Urticaria