J Periodontal Implant Sci.
2017 Dec;47(6):388-401. 10.5051/jpis.2017.47.6.388.
Physicochemical characterization of porcine bone-derived grafting material and comparison with bovine xenografts for dental applications
- Affiliations
-
- 1School of Advanced Materials Science and Engineering, Sungkyunkwan University, Suwon, Korea. jhlee7@skku.edu, kimdj@skku.edu
- 2SKKU Advanced Institute of Nanotechnology, Sungkyunkwan University, Suwon, Korea.
- KMID: 2399745
- DOI: http://doi.org/10.5051/jpis.2017.47.6.388
Abstract
- PURPOSE
The physicochemical properties of a xenograft are very important because they strongly influence the bone regeneration capabilities of the graft material. Even though porcine xenografts have many advantages, only a few porcine xenografts are commercially available, and most of their physicochemical characteristics have yet to be reported. Thus, in this work we aimed to investigate the physicochemical characteristics of a porcine bone grafting material and compare them with those of 2 commercially available bovine xenografts to assess the potential of xenogenic porcine bone graft materials for dental applications.
METHODS
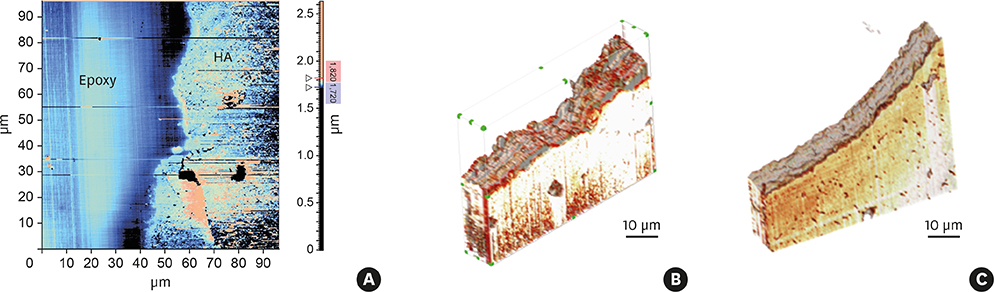
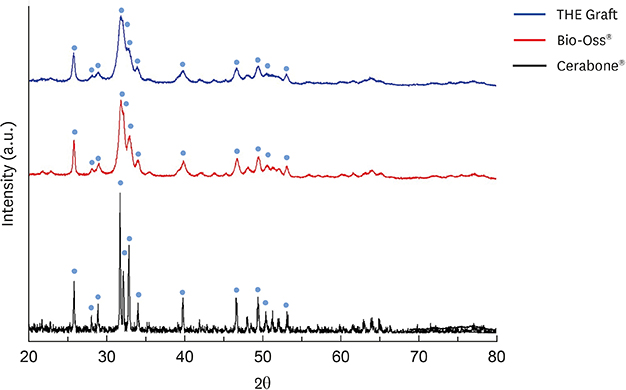
We used various characterization techniques, such as scanning electron microscopy, the Brunauer-Emmett-Teller adsorption method, atomic force microscopy, Fourier-transform infrared spectroscopy, X-ray diffraction, and others, to compare the physicochemical properties of xenografts of different origins.
RESULTS
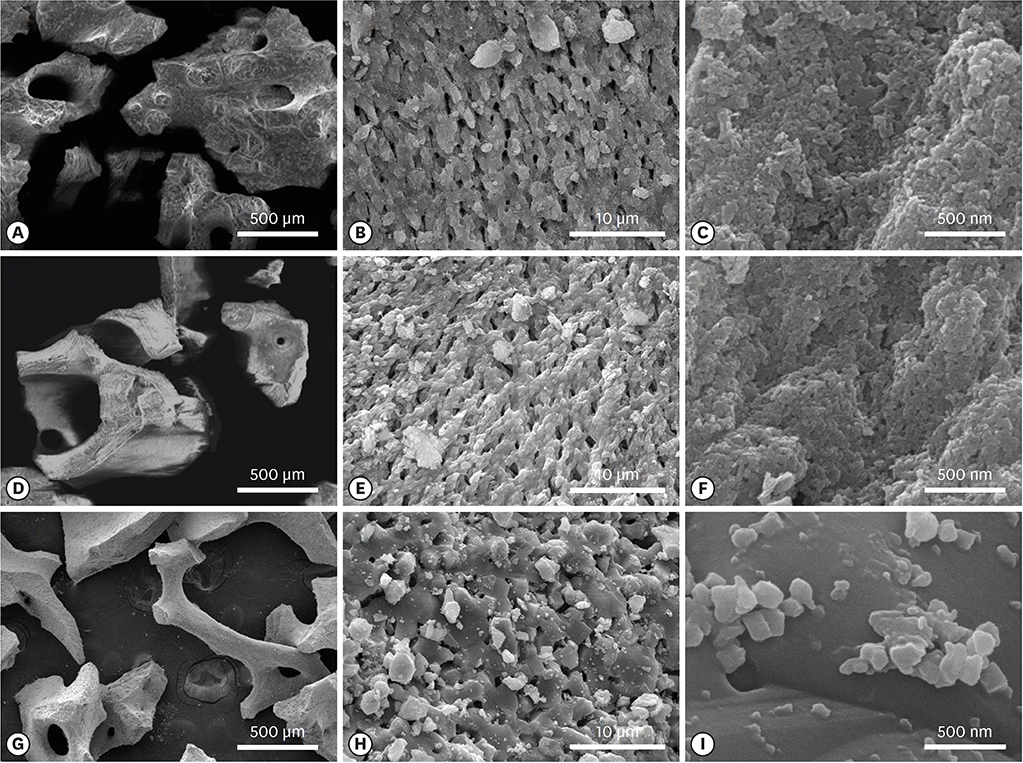
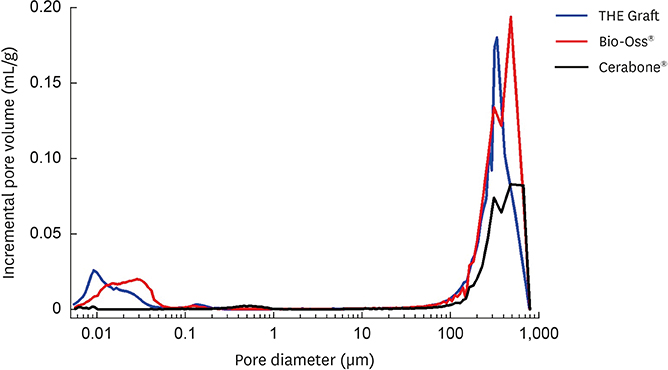
The porcine bone grafting material had relatively high porosity (78.4%) and a large average specific surface area (SSA; 69.9 m²/g), with high surface roughness (10-point average roughness, 4.47 µm) and sub-100-nm hydroxyapatite crystals on the surface. Moreover, this material presented a significant fraction of sub-100-nm pores, with negligible amounts of residual organic substances. Apart from some minor differences, the overall characteristics of the porcine bone grafting material were very similar to those of one of the bovine bone grafting material. However, many of these morphostructural properties were significantly different from the other bovine bone grafting material, which exhibited relatively smooth surface morphology with a porosity of 62.0% and an average SSA of 0.5 m²/g.
CONCLUSIONS
Considering that both bovine bone grafting materials have been successfully used in oral surgery applications in the last few decades, this work shows that the porcine-derived grafting material possesses most of the key physiochemical characteristics required for its application as a highly efficient xenograft material for bone replacement.
MeSH Terms
Figure
Reference
-
1. Park SY, Kim KI, Park SP, Lee JH, Jung HS. Aspartic acid-assisted synthesis of multifunctional strontium-substituted hydroxyapatite microspheres. Cryst Growth Des. 2016; 16:4318–4326.
Article2. Kim DH, Kim KI, Yoon S, Kim HJ, Ahn JS, Jun SH, et al. Dental hetero-graft materials with nano hydroxyapatite surface treatment. J Nanosci Nanotechnol. 2015; 15:7942–7949.3. Richardson CR, Mellonig JT, Brunsvold MA, McDonnell HT, Cochran DL. Clinical evaluation of Bio-Oss®: a bovine-derived xenograft for the treatment of periodontal osseous defects in humans. J Clin Periodontol. 1999; 26:421–428.
Article4. Tadic D, Epple M. A thorough physicochemical characterisation of 14 calcium phosphate-based bone substitution materials in comparison to natural bone. Biomaterials. 2004; 25:987–994.
Article5. Hölzer A, Pietschmann MF, Rösl C, Hentschel M, Betz O, Matsuura M, et al. The interrelation of trabecular microstructural parameters of the greater tubercle measured for different species. J Orthop Res. 2012; 30:429–434.
Article6. Lorenzen E, Follmann F, Jungersen G, Agerholm JS. A review of the human vs. porcine female genital tract and associated immune system in the perspective of using minipigs as a model of human genital Chlamydia infection. Vet Res (Faisalabad). 2015; 46:116.
Article7. Salamanca E, Lee WF, Lin CY, Huang HM, Lin CT, Feng SW, et al. A novel porcine graft for regeneration of bone defects. Materials (Basel). 2015; 8:2523–2536.
Article8. Ramírez-Fernández M, Calvo-Guirado JL, Delgado-Ruiz RA, Maté-Sánchez del Val JE, Vicente-Ortega V, Meseguer-Olmos L. Bone response to hydroxyapatites with open porosity of animal origin (porcine [OsteoBiol®mp3] and bovine [Endobon®]): a radiological and histomorphometric study. Clin Oral Implants Res. 2011; 22:767–773.
Article9. Go A, Kim SE, Shim KM, Lee SM, Choi SH, Son JS, et al. Osteogenic effect of low-temperature-heated porcine bone particles in a rat calvarial defect model. J Biomed Mater Res A. 2014; 102:3609–3617.
Article10. Mochalov KE, Efimov AE, Bobrovsky A, Agapov II, Chistyakov AA, Oleinikov V, et al. Combined scanning probe nanotomography and optical microspectroscopy: a correlative technique for 3D characterization of nanomaterials. ACS Nano. 2013; 7:8953–8962.
Article11. Mochalov KE, Efimov AE, Oleinikov VA, Nabiev I. High-resolution scanning near-field optical nanotomography: a technique for 3D multimodal nanoscale characterization of nano-biophotonic materials. Phys Procedia. 2015; 73:168–172.
Article12. Mariotti F, Tomé D, Mirand PP. Converting nitrogen into protein--beyond 6.25 and Jones' factors. Crit Rev Food Sci Nutr. 2008; 48:177–184.
Article13. Renders GA, Mulder L, van Ruijven LJ, van Eijden TM. Porosity of human mandibular condylar bone. J Anat. 2007; 210:239–248.
Article14. Hing KA, Annaz B, Saeed S, Revell PA, Buckland T. Microporosity enhances bioactivity of synthetic bone graft substitutes. J Mater Sci Mater Med. 2005; 16:467–475.
Article15. Lee DS, Pai Y, Chang S. Physicochemical characterization of InterOss® and Bio-Oss® anorganic bovine bone grafting material for oral surgery: a comparative study. Mater Chem Phys. 2014; 146:99–104.
Article16. Lowell S, Shields JE. Powder surface area and porosity. 3rd ed. New York (NY): Springers;1991.17. Webb PA, Orr C. Analytical methods in fine particle technology. Norcross (GA): Micromeritics Instrument Corporation;1997.18. Lee H, Lee W, Lee JH, Yoon DS. Surface potential analysis of nanoscale biomaterials and devices using kelvin probe force microscopy. J Nanomater. 2016; 2016:4209130.
Article19. Figueiredo M, Henriques J, Martins G, Guerra F, Judas F, Figueiredo H. Physicochemical characterization of biomaterials commonly used in dentistry as bone substitutes--comparison with human bone. J Biomed Mater Res B Appl Biomater. 2010; 92:409–419.
Article20. LeGeros RZ. Calcium phosphates in oral biology and medicine. Monogr Oral Sci. 1991; 15:1–201.21. Berzina-Cimdina L, Borodajenko N. Research of calcium phosphates using Fourier transform infrared spectroscopy. London: INTECH Open Access Publisher;2012.22. Rivera-Muñoz EM. Hydroxyapatite-based materials: synthesis and characterization. In : Fazel-Rezai R, editor. Biomedical engineering: frontiers and challenges. London: INTECH Open Access Publisher;2011. Chapter 4.23. Figueiredo MM, Gamelas JA, Martins AG. Characterization of bone and bone-based graft materials using FTIR spectroscopy. In : Theophanides T, editor. Infrared spectroscopy: life and biomedical sciences. London: INTECH Open Access Publisher;2012. Chapter 18.24. Sáez-Plaza P, Navas MJ, Wybraniec S, Michałowski T, Asuero AG. An overview of the Kjeldahl method of nitrogen determination. Part II. Sample preparation, working scale, instrumental finish, and quality control. Crit Rev Anal Chem. 2013; 43:224–272.
Article25. Cohen SA, Strydom DJ. Amino acid analysis utilizing phenylisothiocyanate derivatives. Anal Biochem. 1988; 174:1–16.
Article26. Crabb JW, West KA, Dodson WS, Hulmes JD. Amino acid analysis. Curr Protoc Protein Sci. 2001; Chapter 11:Unit 11.9.
Article27. Cowin SC. Bone mechanics. Boca Raton (FL): CRC Press;1989.28. da Cruz AC, Pochapski MT, Daher JB, da Silva JC, Pilatti GL, Santos FA. Physico-chemical characterization and biocompatibility evaluation of hydroxyapatites. J Oral Sci. 2006; 48:219–226.
Article29. Murugan R, Ramakrishna S, Panduranga Rao K. Nanoporous hydroxy-carbonate apatite scaffold made of natural bone. Mater Lett. 2006; 60:2844–2847.
Article30. Webster TJ, Ergun C, Doremus RH, Siegel RW, Bizios R. Enhanced functions of osteoblasts on nanophase ceramics. Biomaterials. 2000; 21:1803–1810.
Article31. Li X, van Blitterswijk CA, Feng Q, Cui F, Watari F. The effect of calcium phosphate microstructure on bone-related cells in vitro . Biomaterials. 2008; 29:3306–3316.
Article32. Kubies D, Himmlová L, Riedel T, Chánová E, Balík K, Douděrová M, et al. The interaction of osteoblasts with bone-implant materials: 1. The effect of physicochemical surface properties of implant materials. Physiol Res. 2011; 60:95–111.
Article33. Rupp F, Gittens RA, Scheideler L, Marmur A, Boyan BD, Schwartz Z, et al. A review on the wettability of dental implant surfaces I: theoretical and experimental aspects. Acta Biomater. 2014; 10:2894–2906.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Horizontal ridge augmentation with porcine bone-derived grafting material: a long-term retrospective clinical study with more than 5 years of follow-up
- The literature review on the sinus bone graft using deproteinized bovine bone mineral with lateral approach
- A comparison between anorganic bone and collagen-preserving bone xenografts for alveolar ridge preservation: systematic review and future perspectives
- Comparative scanning electron microscope analysis of the enamel of permanent human, bovine and porcine teeth
- The comparative study: the regenerative effect depends on size of bone graft material in bone loss site around dental implant