Diabetes Metab J.
2017 Dec;41(6):474-485. 10.4093/dmj.2017.41.6.474.
Beneficial Effects of Aerobic Exercise Training Combined with Rosiglitazone on Glucose Metabolism in Otsuka Long Evans Tokushima Fatty Rats
- Affiliations
-
- 1Department of Internal Medicine, Inha University School of Medicine, Incheon, Korea. namms@inha.ac.kr
- 2Qingdao Endocrine and Diabetes Hospital, Qingdao, China.
- 3Department of Biomedical Sciences, Inha University School of Medicine, Incheon, Korea.
- 4Department of Anatomy, Inha University School of Medicine, Incheon, Korea. sunpark@inha.ac.kr
- KMID: 2398088
- DOI: http://doi.org/10.4093/dmj.2017.41.6.474
Abstract
- BACKGROUND
Regular aerobic exercise is essential for the prevention and management of type 2 diabetes mellitus and may be particularly beneficial for those treated with thiazolidinediones, since it may prevent associated weight gain. This study aimed to evaluate the effect of combined exercise and rosiglitazone treatment on body composition and glucose metabolism in obese diabetes-prone animals.
METHODS
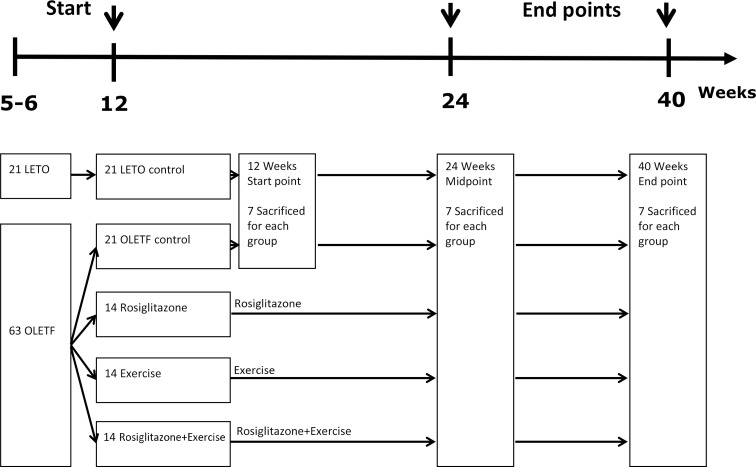
We analyzed metabolic parameters, body composition, and islet profiles in Otsuka Long Evans Tokushima Fatty rats after 28 weeks of aerobic exercise, rosiglitazone treatment, and combined exercise and rosiglitazone treatment.
RESULTS
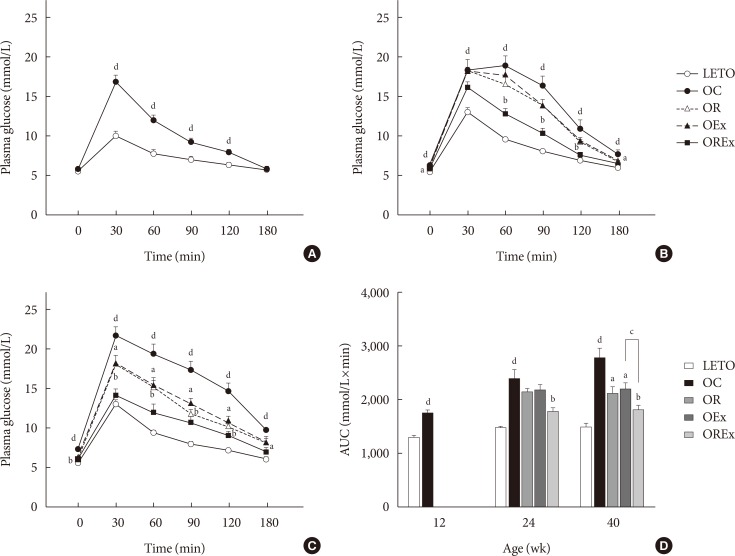
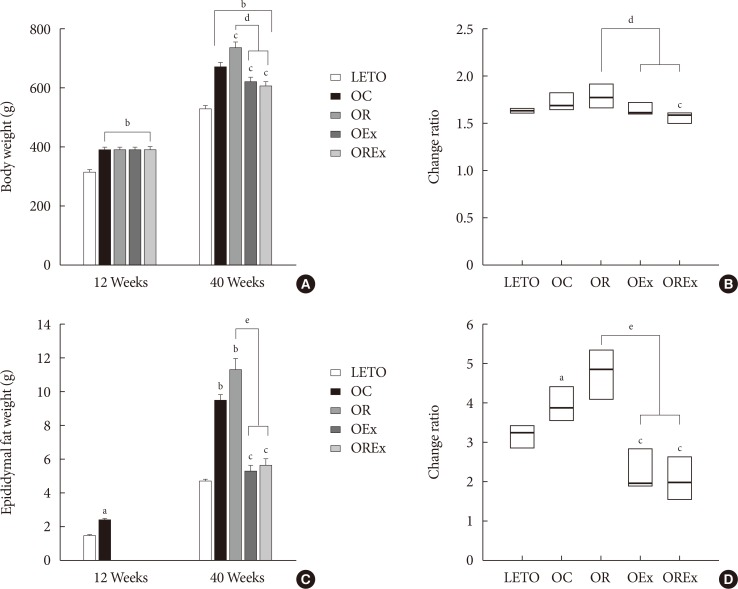
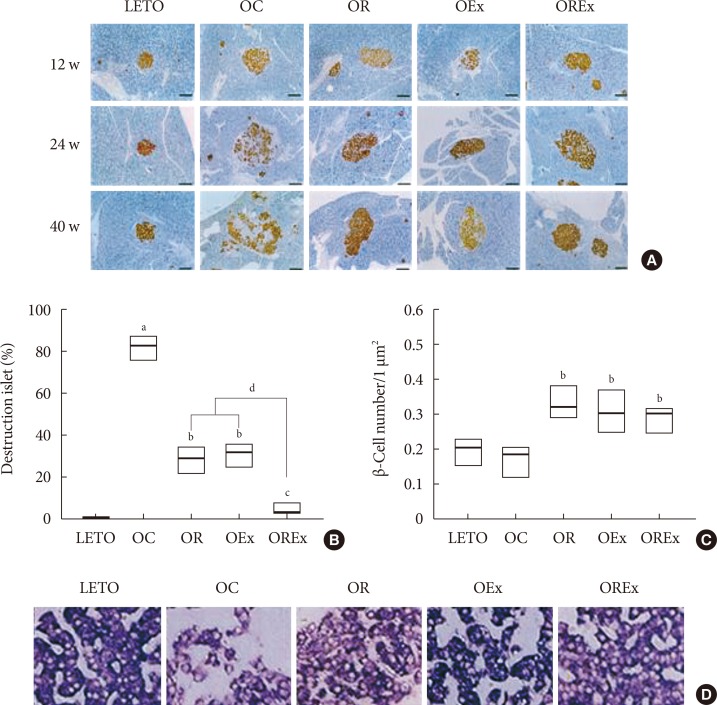
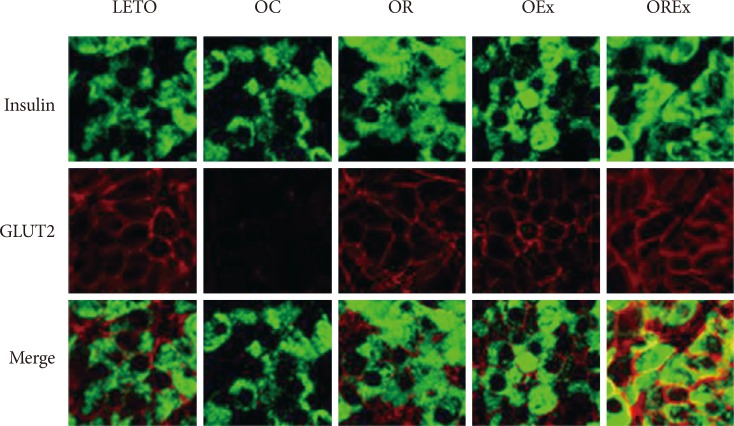
Combined exercise with rosiglitazone showed significantly less increase in weight and epididymal fat compared to rosiglitazone treatment. Aerobic exercise alone and combined rosiglitazone and exercise treatment led to similar retention of lean body mass. All experimental groups showed a decrease in fasting glucose. However, the combined exercise and rosiglitazone therapy group showed prominent improvement in glucose tolerance compared to the other groups. Rescue of islet destruction was observed in all experimental groups, but was most prominent in the combined therapy group.
CONCLUSION
Regular aerobic exercise combined with rosiglitazone treatment can compensate for the adverse effect of rosiglitazone treatment and has benefit for islet preservation.
Keyword
MeSH Terms
Figure
Reference
-
1. Kriketos AD, Carey DG, Jenkins AB, Chisholm DJ, Furler SM, Campbell LV. Central fat predicts deterioration of insulin secretion index and fasting glycaemia: 6-year follow-up of subjects at varying risk of type 2 diabetes mellitus. Diabet Med. 2003; 20:294–300. PMID: 12675643.
Article2. Kahn SE. Clinical review 135: the importance of beta-cell failure in the development and progression of type 2 diabetes. J Clin Endocrinol Metab. 2001; 86:4047–4058. PMID: 11549624.3. Guldstrand M, Ahren B, Adamson U. Improved beta-cell function after standardized weight reduction in severely obese subjects. Am J Physiol Endocrinol Metab. 2003; 284:E557–E565. PMID: 12556352.4. Inzucchi SE, Bergenstal RM, Buse JB, Diamant M, Ferrannini E, Nauck M, Peters AL, Tsapas A, Wender R, Matthews DR. Management of hyperglycaemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetologia. 2012; 55:1577–1596. PMID: 22526604.
Article5. Ferre P. The biology of peroxisome proliferator-activated receptors: relationship with lipid metabolism and insulin sensitivity. Diabetes. 2004; 53(Suppl 1):S43–S50. PMID: 14749265.6. Dana SL, Hoener PA, Bilakovics JM, Crombie DL, Ogilvie KM, Kauffman RF, Mukherjee R, Paterniti JR Jr. Peroxisome proliferator-activated receptor subtype-specific regulation of hepatic and peripheral gene expression in the Zucker diabetic fatty rat. Metabolism. 2001; 50:963–971. PMID: 11474486.
Article7. Etgen GJ, Oldham BA, Johnson WT, Broderick CL, Montrose CR, Brozinick JT, Misener EA, Bean JS, Bensch WR, Brooks DA, Shuker AJ, Rito CJ, McCarthy JR, Ardecky RJ, Tyhonas JS, Dana SL, Bilakovics JM, Paterniti JR Jr, Ogilvie KM, Liu S, Kauffman RF. A tailored therapy for the metabolic syndrome: the dual peroxisome proliferator-activated receptor-alpha/gamma agonist LY465608 ameliorates insulin resistance and diabetic hyperglycemia while improving cardiovascular risk factors in preclinical models. Diabetes. 2002; 51:1083–1087. PMID: 11916929.8. Koh EH, Kim MS, Park JY, Kim HS, Youn JY, Park HS, Youn JH, Lee KU. Peroxisome proliferator-activated receptor (PPAR)-alpha activation prevents diabetes in OLETF rats: comparison with PPAR-gamma activation. Diabetes. 2003; 52:2331–2337. PMID: 12941773.9. Hull RL, Shen ZP, Watts MR, Kodama K, Carr DB, Utzschneider KM, Zraika S, Wang F, Kahn SE. Long-term treatment with rosiglitazone and metformin reduces the extent of, but does not prevent, islet amyloid deposition in mice expressing the gene for human islet amyloid polypeptide. Diabetes. 2005; 54:2235–2244. PMID: 15983227.
Article10. Umrani DN, Banday AA, Hussain T, Lokhandwala MF. Rosiglitazone treatment restores renal dopamine receptor function in obese Zucker rats. Hypertension. 2002; 40:880–885. PMID: 12468573.
Article11. Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, Chasan-Taber L, Albright AL, Braun B. American College of Sports Medicine. American Diabetes Association. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association. Joint position statement. Diabetes Care. 2010; 33:e147–e167. PMID: 21115758.
Article12. American Diabetes Association. Standards of medical care in diabetes: 2015. Summary of revisions. Diabetes Care. 2015; 38(Suppl):S4.13. Kadoglou NP, Iliadis F, Liapis CD, Perrea D, Angelopoulou N, Alevizos M. Beneficial effects of combined treatment with rosiglitazone and exercise on cardiovascular risk factors in patients with type 2 diabetes. Diabetes Care. 2007; 30:2242–2244. PMID: 17586747.
Article14. Kadoglou NP, Iliadis F, Sailer N, Athanasiadou Z, Vitta I, Kapelouzou A, Karayannacos PE, Liapis CD, Alevizos M, Angelopoulou N, Vrabas IS. Exercise training ameliorates the effects of rosiglitazone on traditional and novel cardiovascular risk factors in patients with type 2 diabetes mellitus. Metabolism. 2010; 59:599–607. PMID: 19922961.
Article15. Narendran P, Solomon TP, Kennedy A, Chimen M, Andrews RC. The time has come to test the beta cell preserving effects of exercise in patients with new onset type 1 diabetes. Diabetologia. 2015; 58:10–18. PMID: 25367458.
Article16. Okauchi N, Mizuno A, Zhu M, Ishida K, Sano T, Noma Y, Shima K. Effects of obesity and inheritance on the development of non-insulin-dependent diabetes mellitus in Otsuka-Long-Evans-Tokushima fatty rats. Diabetes Res Clin Pract. 1995; 29:1–10. PMID: 8593753.
Article17. Vanheest JL, Rodgers CD. Effects of exercise in diabetic rats before and during gestation on maternal and neonatal outcomes. Am J Physiol. 1997; 273(4 Pt 1):E727–E733. PMID: 9357802.18. Johns DG, Ao Z, Eybye M, Olzinski A, Costell M, Gruver S, Smith SA, Douglas SA, Macphee CH. Rosiglitazone protects against ischemia/reperfusion-induced leukocyte adhesion in the zucker diabetic fatty rat. J Pharmacol Exp Ther. 2005; 315:1020–1027. PMID: 16123307.
Article19. Muniyappa R, Chen H, Muzumdar RH, Einstein FH, Yan X, Yue LQ, Barzilai N, Quon MJ. Comparison between surrogate indexes of insulin sensitivity/resistance and hyperinsulinemic euglycemic clamp estimates in rats. Am J Physiol Endocrinol Metab. 2009; 297:E1023–E1029. PMID: 19706785.
Article20. Kim SY, Lee SH, Kim BM, Kim EH, Min BH, Bendayan M, Park IS. Activation of nestin-positive duct stem (NPDS) cells in pancreas upon neogenic motivation and possible cytodifferentiation into insulin-secreting cells from NPDS cells. Dev Dyn. 2004; 230:1–11. PMID: 15108304.
Article21. Lee SH, Han YM, Min BH, Park IS. Cytoprotective effects of polyenoylphosphatidylcholine (PPC) on beta-cells during diabetic induction by streptozotocin. J Histochem Cytochem. 2003; 51:1005–1015. PMID: 12871982.22. Saris WH, Blair SN, van Baak MA, Eaton SB, Davies PS, Di Pietro L, Fogelholm M, Rissanen A, Schoeller D, Swinburn B, Tremblay A, Westerterp KR, Wyatt H. How much physical activity is enough to prevent unhealthy weight gain? Outcome of the IASO 1st Stock Conference and consensus statement. Obes Rev. 2003; 4:101–114. PMID: 12760445.
Article23. Kahn SE, Haffner SM, Heise MA, Herman WH, Holman RR, Jones NP, Kravitz BG, Lachin JM, O’Neill MC, Zinman B, Viberti G. ADOPT Study Group. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N Engl J Med. 2006; 355:2427–2443. PMID: 17145742.
Article24. Minami A, Ishimura N, Harada N, Sakamoto S, Niwa Y, Nakaya Y. Exercise training improves acetylcholine-induced endothelium-dependent hyperpolarization in type 2 diabetic rats, Otsuka Long-Evans Tokushima fatty rats. Atherosclerosis. 2002; 162:85–92. PMID: 11947901.
Article25. Schoeller DA, Shay K, Kushner RF. How much physical activity is needed to minimize weight gain in previously obese women? Am J Clin Nutr. 1997; 66:551–556. PMID: 9280172.
Article26. Ross R, Bradshaw AJ. The future of obesity reduction: beyond weight loss. Nat Rev Endocrinol. 2009; 5:319–325. PMID: 19421242.
Article27. Hallsten K, Virtanen KA, Lonnqvist F, Sipila H, Oksanen A, Viljanen T, Ronnemaa T, Viikari J, Knuuti J, Nuutila P. Rosiglitazone but not metformin enhances insulin- and exercise-stimulated skeletal muscle glucose uptake in patients with newly diagnosed type 2 diabetes. Diabetes. 2002; 51:3479–3485. PMID: 12453903.28. Hevener AL, Reichart D, Olefsky J. Exercise and thiazolidinedione therapy normalize insulin action in the obese Zucker fatty rat. Diabetes. 2000; 49:2154–2159. PMID: 11118020.
Article29. Yaspelkis BB 3rd, Lessard SJ, Reeder DW, Limon JJ, Saito M, Rivas DA, Kvasha I, Hawley JA. Exercise reverses high-fat diet-induced impairments on compartmentalization and activation of components of the insulin-signaling cascade in skeletal muscle. Am J Physiol Endocrinol Metab. 2007; 293:E941–E949. PMID: 17623749.
Article30. Lessard SJ, Rivas DA, Chen ZP, Bonen A, Febbraio MA, Reeder DW, Kemp BE, Yaspelkis BB 3rd, Hawley JA. Tissue-specific effects of rosiglitazone and exercise in the treatment of lipid-induced insulin resistance. Diabetes. 2007; 56:1856–1864. PMID: 17440174.
Article31. Simpson KA, Singh MA. Effects of exercise on adiponectin: a systematic review. Obesity (Silver Spring). 2008; 16:241–256. PMID: 18239630.
Article32. Leiter LA. Beta-cell preservation: a potential role for thiazolidinediones to improve clinical care in type 2 diabetes. Diabet Med. 2005; 22:963–972. PMID: 16026359.
Article33. Butler AE, Janson J, Bonner-Weir S, Ritzel R, Rizza RA, Butler PC. Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes. 2003; 52:102–110. PMID: 12502499.34. Finegood DT, McArthur MD, Kojwang D, Thomas MJ, Topp BG, Leonard T, Buckingham RE. Beta-cell mass dynamics in Zucker diabetic fatty rats. Rosiglitazone prevents the rise in net cell death. Diabetes. 2001; 50:1021–1029. PMID: 11334404.35. Calegari VC, Abrantes JL, Silveira LR, Paula FM, Costa JM Jr, Rafacho A, Velloso LA, Carneiro EM, Bosqueiro JR, Boschero AC, Zoppi CC. Endurance training stimulates growth and survival pathways and the redox balance in rat pancreatic islets. J Appl Physiol (1985). 2012; 112:711–718. PMID: 22174407.
Article36. Shima K, Shi K, Mizuno A, Sano T, Ishida K, Noma Y. Exercise training has a long-lasting effect on prevention of non-insulin-dependent diabetes mellitus in Otsuka-Long-Evans-Tokushima Fatty rats. Metabolism. 1996; 45:475–480. PMID: 8609834.
Article37. Slentz CA, Tanner CJ, Bateman LA, Durheim MT, Huffman KM, Houmard JA, Kraus WE. Effects of exercise training intensity on pancreatic beta-cell function. Diabetes Care. 2009; 32:1807–1811. PMID: 19592624.38. Gupta D, Peshavaria M, Monga N, Jetton TL, Leahy JL. Physiologic and pharmacologic modulation of glucose-dependent insulinotropic polypeptide (GIP) receptor expression in beta-cells by peroxisome proliferator-activated receptor (PPAR)-gamma signaling: possible mechanism for the GIP resistance in type 2 diabetes. Diabetes. 2010; 59:1445–1450. PMID: 20332343.39. Meidute Abaraviciene S, Lundquist I, Galvanovskis J, Flodgren E, Olde B, Salehi A. Palmitate-induced beta-cell dysfunction is associated with excessive NO production and is reversed by thiazolidinedione-mediated inhibition of GPR40 transduction mechanisms. PLoS One. 2008; 3:e2182. PMID: 18478115.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Rosiglitazone on Inflammation in Otsuka Long-Evans Tokushima Fatty Rats
- Response: Effects of Rosiglitazone on Inflammation in Otsuka Long-Evans Tokushima Fatty Rats (Korean Diabetes J 2010;34:191-9)
- Effect of Exercise on Glucose Metabolism
- Letter: Effects of Rosiglitazone on Inflammation in Otsuka Long-Evans Tokushima Fatty Rats (Korean Diabetes J 2010;34:191-9)
- Beneficial Effects of Thiazolidinediones on Diabetic Nephropathy in OLETF Rats