J Adv Prosthodont.
2017 Dec;9(6):453-462. 10.4047/jap.2017.9.6.453.
Investigation of the cytotoxicity of thermoplastic denture base resins
- Affiliations
-
- 1Institute of Tissue Regeneration Engineering (ITREN), Dankook University, Cheonan, Republic of Korea. ducious@gmail.com, haelee@dku.edu
- 2Department of Biomaterials Science, College of Dentistry, Dankook University, Cheonan, Republic of Korea.
- 3Department of Removable Prosthodontics, Tsurumi University School of Dental Medicine, Tsurumi-ku, Yokohama-shi, Japan.
- KMID: 2398050
- DOI: http://doi.org/10.4047/jap.2017.9.6.453
Abstract
- PURPOSE
The purpose of this study was to investigate the in vitro cytotoxicity of thermoplastic denture base resins and to identify the possible adverse effects of these resins on oral keratinocytes in response to hot water/food intake.
MATERIALS AND METHODS
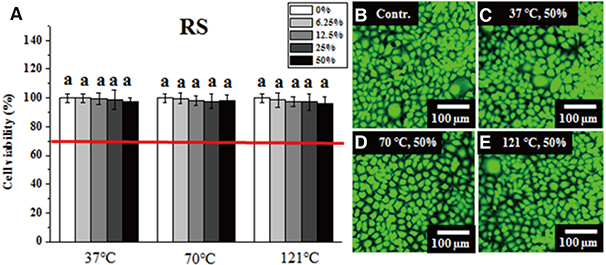
Six dental thermoplastic resin materials were evaluated: three polyamide materials (Smile tone, ST; Valplast, VP; and Luciton FRS, LF), two acrylic materials (Acrytone, AT; and Acryshot, AS), and one polypropylene resin material (Unigum, UG). One heat-polymerized acrylic resin (Vertex RS, RS) was chosen for comparison. After obtaining extracts from specimens of the denture resin materials (Φ=10 mm and d=2 mm) under different extraction conditions (37℃ for 24 hours, 70℃ for 24 hours, and 121℃ for 1 hour), the extracts (50%) or serial dilutions (25%, 12.5%, and 6.25%) in distilled water were co-cultured for 24 hours with immortalized human oral keratinocytes (IHOKs) or mouse fibroblasts (L929s) for the cytotoxicity assay described in ISO 10993.
RESULTS
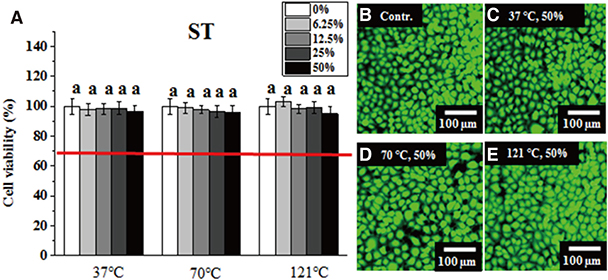
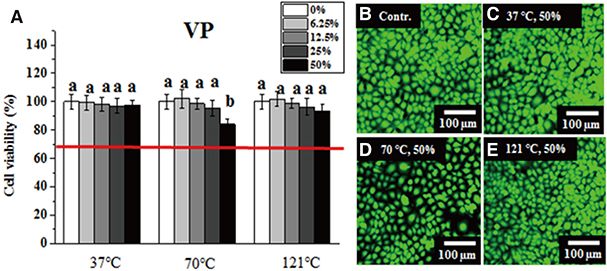
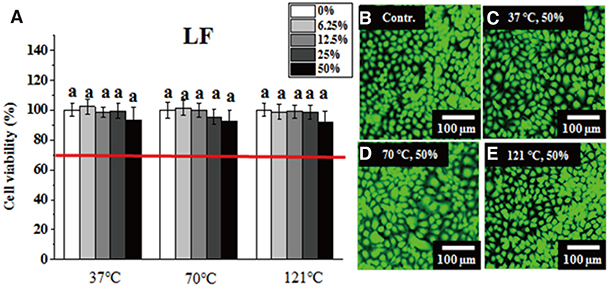
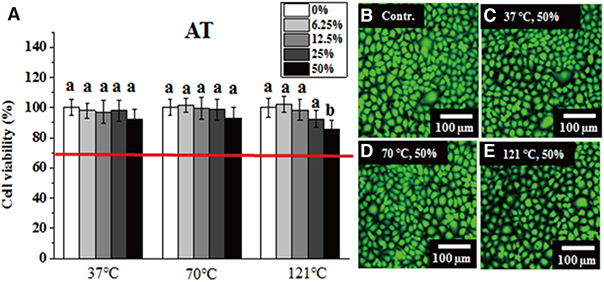
Greater than 70% viability was detected under all test conditions. Significantly lower IHOK and L929 viability was detected in the 50% extract from the VP (70℃) and AT (121℃) samples (P < .05), but only L929 showed reduced viability in the 50% and 25% extract from LF (37℃) (P < .05).
CONCLUSION
Extracts obtained from six materials under different extraction conditions (37℃, 70℃, and 121℃) did not exhibit severe cytotoxicity (less than 70% viability), although their potential risk to oral mucosa at high temperatures should not be ignored.
Keyword
MeSH Terms
Figure
Reference
-
1. Sasaki H, Hamanaka I, Takahashi Y, Kawaguchi T. Effect of reinforcement on the flexural properties of injection-molded thermoplastic denture base resins. J Prosthodont. 2015; 12. 18.2. Fueki K, Ohkubo C, Yatabe M, Arakawa I, Arita M, Ino S, Kanamori T, Kawai Y, Kawara M, Komiyama O, Suzuki T, Nagata K, Hosoki M, Masumi S, Yamauchi M, Aita H, Ono T, Kondo H, Tamaki K, Matsuka Y, Tsukasaki H, Fujisawa M, Baba K, Koyano K, Yatani H. Clinical application of removable partial dentures using thermoplastic resin. Part II: Material properties and clinical features of non-metal clasp dentures. J Prosthodont Res. 2014; 58:71–84.3. Wada J, Fueki K, Yatabe M, Takahashi H, Wakabayashi N. A comparison of the fitting accuracy of thermoplastic denture base resins used in non-metal clasp dentures to a conventional heat-cured acrylic resin. Acta Odontol Scand. 2015; 73:33–37.4. Fueki K, Ohkubo C, Yatabe M, Arakawa I, Arita M, Ino S, Kanamori T, Kawai Y, Kawara M, Komiyama O, Suzuki T, Nagata K, Hosoki M, Masumi S, Yamauchi M, Aita H, Ono T, Kondo H, Tamaki K, Matsuka Y, Tsukasaki H, Fujisawa M, Baba K, Koyano K, Yatani H. Clinical application of removable partial dentures using thermoplastic resin-part I: definition and indication of non-metal clasp dentures. J Prosthodont Res. 2014; 58:3–10.5. Ucar Y, Akova T, Aysan I. Mechanical properties of polyamide versus different PMMA denture base materials. J Prosthodont. 2012; 21:173–176.6. Basavaraj E, Ramaraj B, Lee JH. Polyamide 6/carbon black/molybdenum disulphide composites: Friction, wear and morphological characteristics. Mater Chem Phys. 2013; 138:658–665.7. Xu X, He Z, Lu S, Guo D, Yu J. Enhanced thermal and mechanical properties of lignin/polypropylene wood-plastic composite by using flexible segment-containing reactive compatibilizer. Macromol Res. 2014; 22:1084–1089.8. Kim JH, Choe HC, Son MK. Evaluation of adhesion of reline resins to the thermoplastic denture base resin for nonmetal clasp denture. Dent Mater J. 2014; 33:32–38.9. Hamanaka I, Takahashi Y, Shimizu H. Mechanical properties of injection-molded thermoplastic denture base resins. Acta Odontol Scand. 2011; 69:75–79.10. Takabayashi Y. Characteristics of denture thermoplastic resins for non-metal clasp dentures. Dent Mater J. 2010; 29:353–361.11. Iwata Y. Assessment of clasp design and flexural properties of acrylic denture base materials for use in non-metal clasp dentures. J Prosthodont Res. 2016; 60:114–122.12. Jang DE, Lee JY, Jang HS, Lee JJ, Son MK. Color stability, water sorption and cytotoxicity of thermoplastic acrylic resin for non metal clasp denture. J Adv Prosthodont. 2015; 7:278–287.13. Uzun IH, Tatar A, Hacimuftuoglu A, Saruhan F, Bayindir F. In vitro evaluation of long-term cytotoxic response of injection-molded polyamide and polymethyle metacrylate denture base materials on primary fibroblast cell culture. Acta Odontol Scand. 2013; 71:1267–1272.14. Sahin O, Ozdemir AK, Turgut M, Boztug A, Sumer Z. Investigation of flexural strength and cytotoxicity of acrylic resin copolymers by using different polymerization methods. J Adv Prosthodont. 2015; 7:98–107.15. ISO 10993-12. Biological evaluation of medical devices-part 12: sample preparation and reference materials. 3rd ed. Geneva, Switzerland: International Organization for Standardization;2012.16. ISO 7405. Dentistry-evaluation of biocompatibility of medical devices used in dentistry. 3rd ed. Geneva, Switzerland: International Organization for Standardization;2008.17. Black J, Hastings G. Handbook of biomaterial properties. New York, NY: Springer Science & Business Media;2013.18. ISO 10993-5. Biological evaluation of medical devices-part 5: tests for in vitro cytotoxicity. 3rd ed. Geneva, Switzerland: International Organization for Standardization;2009.19. Lee JH, Kwon JS, Moon SK, Uhm SH, Choi BH, Joo UH, Kim KM, Kim KN. Titanium-silver alloy miniplates for mandibular fixation: In vitro and in vivo study. J Oral Maxillofac Surg. 2016; 74:1622.20. Oda D, Bigler L, Lee P, Blanton R. HPV immortalization of human oral epithelial cells: a model for carcinogenesis. Exp Cell Res. 1996; 226:164–169.21. Lee JH, Lee HH, Kim KN, Kim KM. Cytotoxicity and antiinflammatory effects of zinc ions and eugenol during setting of ZOE in immortalized human oral keratinocytes grown as three-dimensional spheroids. Dent Mater. 2016; 32:e93–e104.22. Lee JH, Lee HH, Kim HW, Yu JW, Kim KN, Kim KM. Immunomodulatory/anti-inflammatory effect of ZOE-based dental materials. Dent Mater. 2017; 33:e1–e12.23. Malkoç MA, Demİr N, Şengün A, Bozkurt ŞB, Hakki SS. Cytotoxicity of temporary cements on bovine dental pulp-derived cells (bDPCs) using realtime cell analysis. J Adv Prosthodont. 2015; 7:21–26.24. Nishi C, Nakajima N, Ikada Y. In vitro evaluation of cytotoxicity of diepoxy compounds used for biomaterial modification. J Biomed Mater Res. 1995; 29:829–834.25. Liu X, Xi T, Zheng Y. Influence of the extraction parameters on the cytotoxicity test results of Mg materials. Progress Natural Sci. 2014; 24:507–515.26. dos Santos RL, Pithon MM, Martins FO, Romanos MT, Ruellas AC. Evaluation of cytotoxicity and degree of conversion of glass ionomer cements reinforced with resin. Eur J Orthod. 2012; 34:362–366.27. Miletić I, Devcić N, Anić I, Borcić J, Karlović Z, Osmak M. The cytotoxicity of RoekoSeal and AH plus compared during different setting periods. J Endod. 2005; 31:307–309.28. Cavalcanti BN, Rode SM, Marques MM. Cytotoxicity of substances leached or dissolved from pulp capping materials. Int Endod J. 2005; 38:505–509.29. Kwon JS, Lee SB, Kim CK, Kim KN. Modified cytotoxicity evaluation of elastomeric impression materials while polymerizing with reduced exposure time. Acta Odontol Scand. 2012; 70:597–602.30. Baek HS, Yoo JY, Rah DK, Han DW, Lee DH, Kwon OH, Park JC. Evaluation of the extraction method for the cytotoxicity testing of latex gloves. Yonsei Med J. 2005; 46:579–583.31. Faria AC, Rodrigues RC, Antunes RP, de Mattos Mda G, Rosa AL, Ribeiro RF. Effect of temperature variation on the cytotoxicity of cast dental alloys and commercially pure titanium. J Appl Oral Sci. 2009; 17:421–426.32. Das GK, Chan PP, Teo A, Loo JS, Anderson JM, Tan TT. In vitro cytotoxicity evaluation of biomedical nanoparticles and their extracts. J Biomed Mater Res A. 2010; 93:337–346.33. Goiato MC, Freitas E, dos Santos D, de Medeiros R, Sonego M. Acrylic resin cytotoxicity for denture base-literature review. Adv Clin Exp Med. 2015; 24:679–686.34. Hensten-Pettersen A, Wictorin L. The cytotoxic effect of denture base polymers. Acta Odontol Scand. 1981; 39:101–106.35. Wilkes CE, Summers JW, Daniels CA, Berard MT. PVC handbook. Cincinnati, OH: Hanser Publications;2005.36. Vihola H, Laukkanen A, Valtola L, Tenhu H, Hirvonen J. Cytotoxicity of thermosensitive polymers poly(N-isopropylacrylamide), poly(N-vinylcaprolactam) and amphiphilically modified poly(N-vinylcaprolactam). Biomaterials. 2005; 26:3055–3064.37. Akin H, Tugut F, Polat ZA. In vitro comparison of the cytotoxicity and water sorption of two different denture base systems. J Prosthodont. 2015; 24:152–155.38. Browne RM. The in vitro assessment of the cytotoxicity of dental materials-does it have a role? Int Endod J. 1988; 21:50–58.39. O'Brien J, Wilson I, Orton T, Pognan F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur J Biochem. 2000; 267:5421–5426.40. Coulter JA, Jain S, Butterworth KT, Taggart LE, Dickson GR, McMahon SJ, Hyland WB, Muir MF, Trainor C, Hounsell AR, O'Sullivan JM, Schettino G, Currell FJ, Hirst DG, Prise KM. Cell type-dependent uptake, localization, and cytotoxicity of 1.9 nm gold nanoparticles. Int J Nanomedicine. 2012; 7:2673–2685.41. Borenfreund E, Puerner JA. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol Lett. 1985; 24:119–124.42. Lee JH, Seo SH, Lee SB, Om JY, Kim KM, Kim KN. Cytotoxicity and terminal differentiation of human oral keratinocyte by indium ions from a silver-palladium-gold-indium dental alloy. Dent Mater. 2015; 31:123–133.43. Lee TC, Ho IC. Differential cytotoxic effects of arsenic on human and animal cells. Environ Health Perspect. 1994; 102:101–105.44. Lovschall H, Eiskjaer M, Arenholt-Bindslev D. Formaldehyde cytotoxicity in three human cell types assessed in three different assays. Toxicol In Vitro. 2002; 16:63–69.45. Schweikl H, Schmalz G. Toxicity parameters for cytotoxicity testing of dental materials in two different mammalian cell lines. Eur J Oral Sci. 1996; 104:292–299.46. Kwon JS, Illeperuma RP, Kim J, Kim KM, Kim KN. Cytotoxicity evaluation of zinc oxide-eugenol and non-eugenol cements using different fibroblast cell lines. Acta Odontol Scand. 2014; 72:64–70.47. Chen F, Wu T, Cheng X. Cytotoxic effects of denture adhesives on primary human oral keratinocytes, fibroblasts and permanent L929 cell lines. Gerodontology. 2014; 31:4–10.48. Wataha JC, Rueggeberg FA, Lapp CA, Lewis JB, Lockwood PE, Ergle JW, Mettenburg DJ. In vitro cytotoxicity of resincontaining restorative materials after aging in artificial saliva. Clin Oral Investig. 1999; 3:144–149.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Color stability, water sorption and cytotoxicity of thermoplastic acrylic resin for non metal clasp denture
- A Study on the Bond Strength of Reline Resin to Pressure Injection Type Thermoplastic Denture Base Resin
- Physical properties and color stability of injection-molded thermoplastic denture base resins
- Cytotoxicity Of Denture Base Resins
- Comparison of flexural strength according to thickness between CAD/CAM denture base resins and conventional denture base resins