Ann Hepatobiliary Pancreat Surg.
2017 Nov;21(4):205-211. 10.14701/ahbps.2017.21.4.205.
Assessing the role of everolimus in reducing hepatocellular carcinoma recurrence after living donor liver transplantation for patients within the UCSF criteria: re-inventing the role of mammalian target of rapamycin inhibitors
- Affiliations
-
- 1Organ Transplantation Center, China Medical University Hospital, Taichung, Taiwan. otc@mail.cmuh.org.tw
- 2Department of Surgery, China Medical University Hospital, Taichung, Taiwan.
- 3Department of Anaesthesiology, China Medical University Hospital, Taichung, Taiwan.
- KMID: 2397800
- DOI: http://doi.org/10.14701/ahbps.2017.21.4.205
Abstract
- BACKGROUNDS/AIMS
The protective effect of everolimus (EVR) in hepatocellular carcinoma (HCC) patients who receive liver transplantation in terms of reducing the recurrence has not been sufficiently investigated in clinical trials. In this second stage of our ongoing study, we intend to analyze the effects of EVR as an immunosuppressant, when it is started in the early phase after living donor liver transplantation (LDLT), on HCC recurrence in patients with HCC within the University of California at San Francisco (UCSF) criteria.
METHODS
From January 2011 to June 2013, a total of 250 patients underwent LDLT for HCC at our institute. The patients with HCC within the UCSF criteria were included in the study and divided in two groups depending upon the postoperative immunosuppression. Group A: HCC patients that received EVR+TAC based immunosuppressive regimen (n=37). Group B: HCC patients that received standard TAC based immunosuppressive regimen without EVR (n=29). The target trough level for EVR was 3 to 5 ng/ml while for TAC it was 8-10 ng/ml.
RESULTS
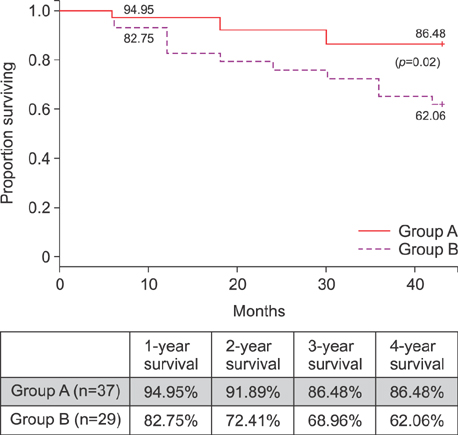
For group A patients, the mean trough level of the EVR was 3.47±1.53 ng/ml (range, 1.5-11.2) with a daily dose of 1.00±0.25 mg/day. For group A and B, the average TAC trough levels were 6.97±3.98 ng/ml (range, 2.50 to 11.28 ng/ml) and 6.93±2.58 (range, 2-16.30), respectively. The 1-year, 3-year and 4-year overall survival achieved for Group A patients was 94.95%, 86.48% and 86.48%, respectively while for Group B patients it was 82.75%, 68.96%, and 62.06%, respectively (p=0.0217).
CONCLUSIONS
EVR use in liver transplant recipients in the early stage after transplantation reduces the HCC recurrence rates in HCC patients within the UCSF criteria.
Keyword
MeSH Terms
Figure
Reference
-
1. Mazzaferro V, Regalia E, Doci R, Andreola S, Pulvirenti A, Bozzetti F, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996; 334:693–699.2. Yao FY, Ferrell L, Bass NM, Watson JJ, Bacchetti P, Venook A, et al. Liver transplantation for hepatocellular carcinoma: expansion of the tumor size limits does not adversely impact survival. Hepatology. 2001; 33:1394–1403.3. Belghiti J, Durand F. Criteria for liver transplantation for hepatocellular carcinoma: what is an acceptable outcome? Liver Int. 2011; 31:Suppl 1. 161–163.4. Jeng LB, Thorat A, Yang HR, Yeh CC, Chen TH, Hsu CH, et al. Successful use of hepatitis B surface antigen-positive liver grafts - an effective source for donor organs in endemic areas: a single-center experience. Ann Transplant. 2015; 20:103–111.5. Marsh JW, Dvorchik I. Liver organ allocation for hepatocellular carcinoma: are we sure? Liver Transpl. 2003; 9:693–696.6. Toso C, Trotter J, Wei A, Bigam DL, Shah S, Lancaster J, et al. Total tumor volume predicts risk of recurrence following liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2008; 14:1107–1115.7. Mazzaferro V, Llovet JM, Miceli R, Bhoori S, Schiavo M, Mariani L, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol. 2009; 10:35–43.8. Chan SC, Fan ST, Chok KS, Cheung TT, Chan AC, Fung JY, et al. Survival advantage of primary liver transplantation for hepatocellular carcinoma within the up-to-7 criteria with microvascular invasion. Hepatol Int. 2011; 6:646–656.9. Lei JY, Wang WT, Yan LN. Up-to-seven criteria for hepatocellular carcinoma liver transplantation: a single center analysis. World J Gastroenterol. 2013; 19:6077–6083.10. Ferreiro AO, Vazquez-Millán MA, López FS, Gutiérrez MG, Diaz SP, Patiño MJ. Everolimus-based immunosuppression in patients with hepatocellular carcinoma at high risk of recurrence after liver transplantation: a case series. Transplant Proc. 2014; 46:3496–3501.11. Zimmerman MA, Trotter JF, Wachs M, Bak T, Campsen J, Skibba A, et al. Sirolimus-based immunosuppression following liver transplantation for hepatocellular carcinoma. Liver Transpl. 2008; 14:633–638.12. Penn I, Starzl TE. Malignant tumors arising de novo in immunosuppressed organ transplant recipients. Transplantation. 1972; 14:407–417.13. Aberg F, Pukkala E, Höckerstedt K, Sankila R, Isoniemi H. Risk of malignant neoplasms after liver transplantation: a population-based study. Liver Transpl. 2008; 14:1428–1436.14. Hojo M, Morimoto T, Maluccio M, Asano T, Morimoto K, Lagman M, et al. Cyclosporine induces cancer progression by a cell-autonomous mechanism. Nature. 1999; 397:530–534.15. Maluccio M, Sharma V, Lagman M, Vyas S, Yang H, Li B, et al. Tacrolimus enhances transforming growth factor-beta1 expression and promotes tumor progression. Transplantation. 2003; 76:597–602.16. Vivarelli M, Cucchetti A, Piscaglia F, La Barba G, Bolondi L, Cavallari A, et al. Analysis of risk factors for tumor recurrence after liver transplantation for hepatocellular carcinoma: key role of immunosuppression. Liver Transpl. 2005; 11:497–503.17. Schumacher G, Oidtmann M, Rosewicz S, Langrehr J, Jonas S, Mueller AR, et al. Sirolimus inhibits growth of human hepatoma cells in contrast to tacrolimus which promotes cell growth. Transplant Proc. 2002; 34:1392–1393.18. Jeng LB, Thorat A, Hsieh YW, Yang HR, Yeh CC, Chen TH, et al. Experience of using everolimus in the early stage of living donor liver transplantation. Transplant Proc. 2014; 46:744–748.19. Moini M, Schilsky ML, Tichy EM. Review on immunosuppression in liver transplantation. World J Hepatol. 2015; 7:1355–1368.20. Rodríguez-Perálvarez M, Tsochatzis E, Naveas MC, Pieri G, García-Caparrós C, O'Beirne J, et al. Reduced exposure to calcineurin inhibitors early after liver transplantation prevents recurrence of hepatocellular carcinoma. J Hepatol. 2013; 59:1193–1199.21. Finn RS. Current and future treatment strategies for patients with advanced hepatocellular carcinoma: Role of mTOR inhibition. Liver Cancer. 2012; 1:247–256.22. Wiesner R, Klintmalm G, McDiarmid S;. Sirolimus immunotherapy results in reduced rates of acute rejection in de novo orthotopic liver transplant recipients. Am J Transplant. 2002; 2:464. Abstract 1294.23. Liang W, Wang D, Ling X, Kao AA, Kong Y, Shang Y, et al. Sirolimus-based immunosuppression in liver transplantation for hepatocellular carcinoma: a meta-analysis. Liver Transpl. 2012; 18:62–69.24. Saliba F, Dharancy S, Lorho R, Conti F, Radenne S, Neau-Cransac M, et al. Conversion to everolimus in maintenance liver transplant patients: a multicenter, retrospective analysis. Liver Transpl. 2011; 17:905–913.25. Fischer L, Klempnauer J, Beckebaum S, Metselaar HJ, Neuhaus P, Schemmer P, et al. A randomized, controlled study to assess the conversion from calcineurin-inhibitors to everolimus after liver transplantation--PROTECT. Am J Transplant. 2012; 12:1855–1865.26. Levy G, Schmidli H, Punch J, Tuttle-Newhall E, Mayer D, Neuhaus P, et al. Safety, tolerability, and efficacy of everolimus in de novo liver transplant recipients: 12- and 36-month results. Liver Transpl. 2006; 12:1640–1648.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Long term outcome of hepatocellular carcinoma managed with an elective living donor liver transplantation strategy in high volume center
- The impact of applying University of California San Francisco criteria to patients underwent liver transplantation for hepatocellular carcinoma in a low volume center
- Liver Transplantation for Advanced Hepatocellular Carcinoma
- Indication and Outcome of Liver Transplantation In Patients with Hepatocellular Carcinoma
- Kaposi sarcoma of a liver graft in living donor liver transplantation: a rare case report