Asia Pac Allergy.
2012 Jan;2(1):35-44. 10.5415/apallergy.2012.2.1.35.
IgE cross-reactivity between house dust mite allergens and Ascaris lumbricoides antigens
- Affiliations
-
- 1Department of Biological Sciences, College of Science, University of Santo Tomas, Manila 1015, Philippines. gvalmonte@mnl.ust.edu.ph
- 2The Graduate School, University of Santo Tomas, Manila 1015, Philippines.
- 3Research Center for the Natural and Applied Sciences, University of Santo Tomas, Manila 1015, Philippines.
- KMID: 2397409
- DOI: http://doi.org/10.5415/apallergy.2012.2.1.35
Abstract
- BACKGROUND
Common antigens between intestinal parasites and environmental allergens may play a role in the modulation of allergic immune responses. There is a growing interest in investigating cross-reactivity between common helminths and dust mites affecting humans, particularly in the tropics.
OBJECTIVE
This study examined the cross-reactivity between the human roundworm Ascaris lumbricoides (Al) and three house dust mite (HDM) species.
METHODS
Specific serum IgE levels to HDM species Blomia tropicalis (Bt), Dermatophagoides pteronyssinus (Dp), and Dermatophagoides farinae (Df ); and Al extracts among allergic (n=100) and ascariasis (n=60) subjects were measured through enzyme-linked immunosorbent assay (ELISA). IgE-reactive components of HDM and Al extracts were detected through Western-Blot Analysis. Cross-reactivity between HDMs and Al was determined by ELISA inhibition using HDM and Al-specific sera from allergic (n=15) and ascariasis (n=15) subjects. The IgE-binding capacity of a recombinant paramyosin peptide (Blo t 11-fD) to allergic (n=50) and ascariasis (n=50) subjects' sera were likewise determined.
RESULTS
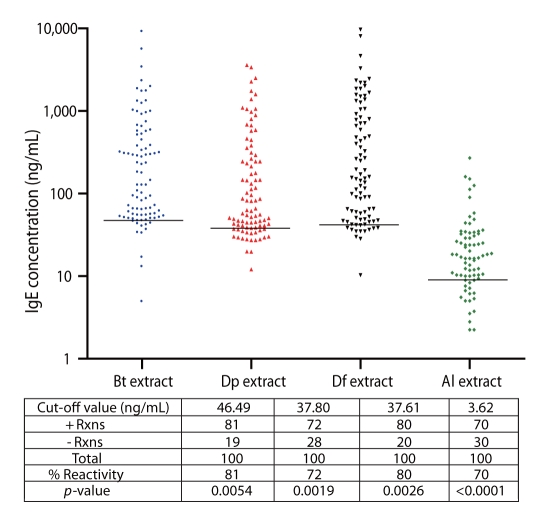
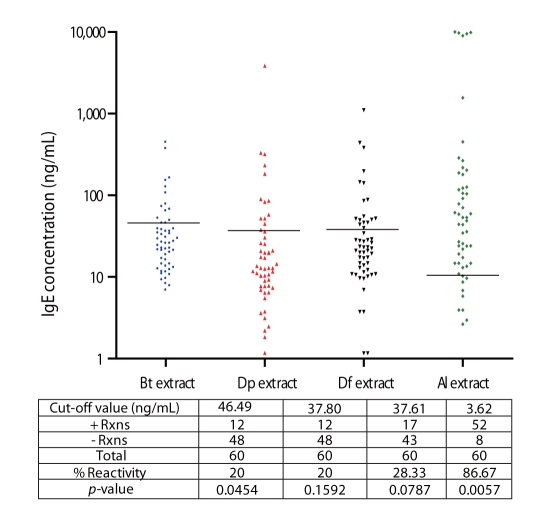
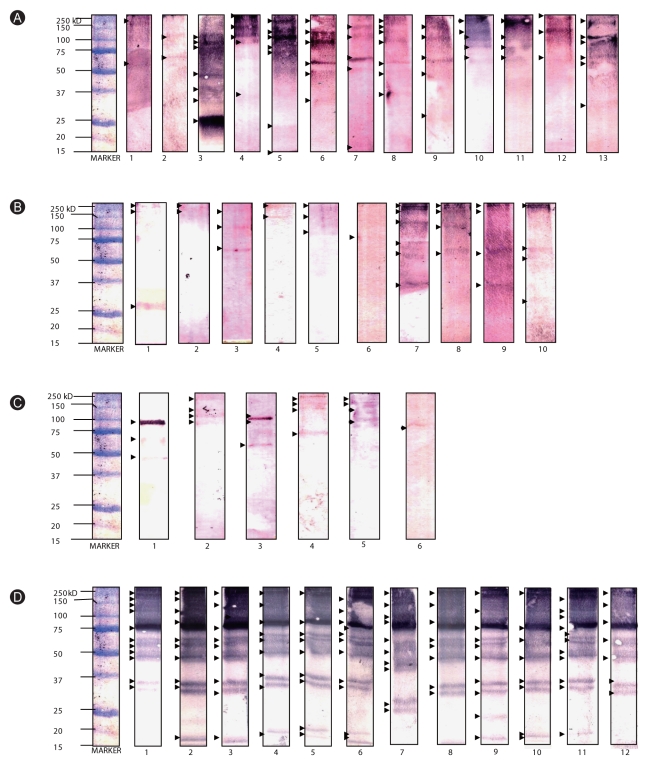
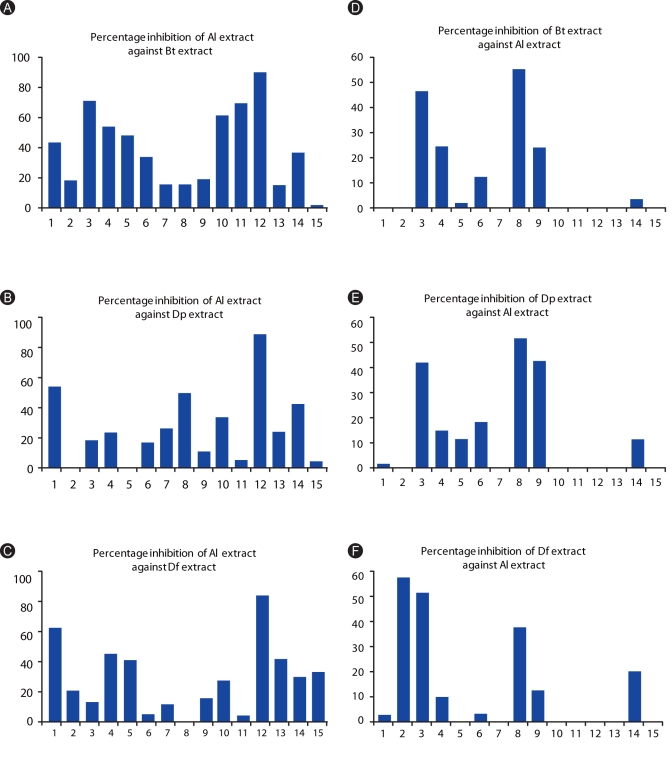
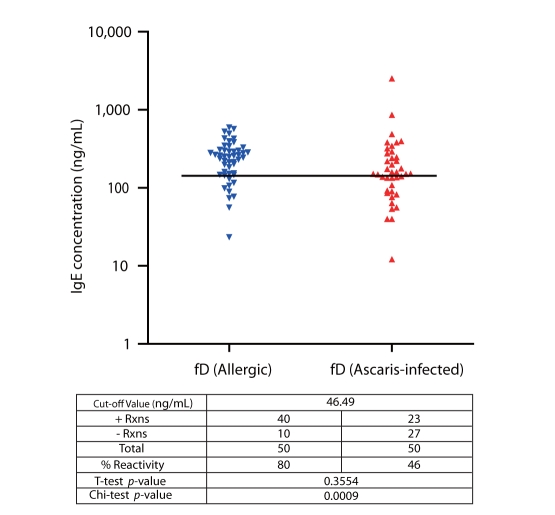
Among allergic subjects, 70% exhibited Al-specific positive IgE-reactivity, while 20-28% of ascariasis subjects demonstrated HDM-specific positive IgE-reactivity. Multiple IgE-reactive components of HDM allergens (14-240 kDa) and Al antigens (15-250 kDa) were detected, indicating multi-allergen sensitization among the subjects tested. Al antigens can inhibit up to 92% of HDM-specific IgE-reactivity among allergic subjects, while up to 54% of Al-specific IgE-reactivity among ascariasis subjects was inhibited by HDM allergens. Positive rBlo t 11-fD-specific IgE reactivity was observed in 80% of the allergic subjects and 46% of the ascariasis subjects.
CONCLUSIONS
This study showed the presence of multiple cross-reactive antigens in HDM and Al extracts. Identification of these molecules may provide basis for designing novel diagnostic and therapeutic strategies. The potential role of paramyosin as a specific cross-reactive allergen present in HDMs and Al has been shown.
Keyword
MeSH Terms
Figure
Reference
-
1. Cooper PJ. Intestinal worms and human allergy. Parasite Immunol. 2004; 26:455–467. PMID: 15771681.
Article2. Flohr C. Dirt, worms and atopic dermatitis. Br J Dermatol. 2003; 148:871–877. PMID: 12786815.
Article3. Gold DR, Wright R. Population disparities in asthma. Annu Rev Public Health. 2005; 26:89–113. PMID: 15760282.
Article4. Hunninghake GM, Soto-Quiros ME, Avila L, Ly NP, Liang C, Sylvia JS, Klanderman BJ, Silverman EK, Celedón JC. Sensitization to Ascaris lumbricoides and severity of childhood asthma in Costa Rica. J Allergy Clin Immunol. 2007; 119:654–661. PMID: 17336615.
Article5. Dagoye D, Bekele Z, Woldemichael K, Nida H, Yimam M, Hall A, Venn AJ, Britton JR, Hubbard R, Lewis SA. Wheezing, allergy, and parasite infection in children in urban and rural Ethiopia. Am J Respir Crit Care Med. 2003; 167:1369–1373. PMID: 12738598.
Article6. Flohr C, Tuyen LN, Lewis S, Quinnell R, Minh TT, Liem HT, Campbell J, Pritchard D, Hien TT, Farrar J, Williams H, Britton J. Poor sanitation and helminth infection protect against skin sensitization in Vietnamese children: A cross-sectional study. J Allergy Clin Immunol. 2006; 118:1305–1311. PMID: 17157661.
Article7. Schäfer T, Meyer T, Ring J, Wichmann HE, Heinrich J. Worm infestation and the negative association with eczema (atopic/nonatopic) and allergic sensitization. Allergy. 2005; 60:1014–1020. PMID: 15969681.
Article8. Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002; 296:490–494. PMID: 11964470.
Article9. Arlian LG, Morgan MS, Neal JS. Dust mite allergens: ecology and distribution. Curr Allergy Asthma Rep. 2002; 2:401–411. PMID: 12165207.
Article10. Arruda LK, Santos AB. Immunologic responses to common antigens in helminthic infections and allergic disease. Curr Opin Allergy Clin Immunol. 2005; 5:399–402. PMID: 16131913.
Article11. Muto R, Imai S, Tezuka H, Furuhashi Y, Fujita K. The biological activity of ABA-1-like protein from Ascaris lumbricoides. J Med Dent Sci. 2001; 48:95–104. PMID: 12160220.12. Pearce EJ, James SL, Hieny S, Lanar DE, Sher A. Induction of protective immunity against Schistosoma mansoni by vaccination with schistosome paramyosin (Sm97), a nonsurface parasite antigen. Proc Natl Acad Sci U S A. 1988; 85:5678–5682. PMID: 3135553.
Article13. Liebau E, Eckelt VH, Wildenburg G, Teesdale-Spittle P, Brophy PM, Walter RD, Henkle-Dührsen K. Structural and functional analysis of a glutathione S-transferase from Ascaris suum. Biochem J. 1997; 324:659–666. PMID: 9182731.
Article14. Johansson E, Aponno M, Lundberg M, Van Hage-Hamsten M. Allergenic cross-reactivity between the nematode Anisakis simplex and the dust mites Acarus siro, Lepidoglyphus destructor, Tyrophagus putrescentiae, and Dermatophagoides pteronyssinus. Allergy. 2001; 56:660–666. PMID: 11421925.
Article15. Ramos JD, Cheong N, Lee BW, Chua KY. Peptide mapping of immunoglobulin E and immunoglobulin G immunodominant epitopes of an allergenic Blomia tropicalis paramyosin, Blo t 11. Clin Exp Allergy. 2003; 33:511–517. PMID: 12680869.
Article16. Salvador-Tayag F, Sumpaico MR. Aeroallergen sensitization and serum immunoglobulin levels of Filipino children with chronic and recurrent otitis media. Philippine J Allergy Asthma Immunol. 2003; 9:8–16.17. Medeiros D, Silva A, Rizzo J, Motta M, Oliveira F, Sarinho E. Total IgE level in respiratory allergy: study of patients at high risk for helminthic infection. J Pediatr (Rio J). 2006; 82:255–259. PMID: 16858505.
Article18. Thomas WR, Smith WA, Hales BJ, Mills KL, O'Brien RM. Characterization and immunobiology of house dust mite allergens. Int Arch Allergy Immunol. 2002; 129:1–18. PMID: 12372994.
Article19. Johansson E, Schmidt M, Johansson SG, Machado L, Olsson S, van Hage-Hamsten M. Allergenic crossreactivity between Lepidoglyphus destructor and Blomia tropicalis. Clin Exp Allergy. 1997; 27:691–699. PMID: 9208191.
Article20. Simpson A, Green R, Custovic A, Woodcock A, Arruda LK, Chapman MD. Skin test reactivity to natural and recombinant Blomia and Dermatophagoides spp. allergens among mite allergic patients in the UK. Allergy. 2003; 58:53–56. PMID: 12580807.
Article21. Thomas WR, Hales BJ, Smith W. Blomia tropicalis: more than just another source of mite allergens. Clin Exp Allergy. 2003; 33:416–418. PMID: 12680854.
Article22. Lozano MJ, Martín HL, Díaz SV, Mañas AI, Valero LA, Campos BM. Cross-reactivity between antigens of Anisakis simplex s.l. and other ascarid nematodes. Parasite. 2004; 11:219–223. PMID: 15224584.23. Ishida MM, Rubinsky-Elefant G, Ferreira AW, Hoshino-Shimizu S, Vaz AJ. Helminth antigens (Taenia solium, Taenia crassiceps, Toxocara canis, Schistosoma mansoni and Echinococcus granulosus) and cross-reactivities in human infections and immunized animals. Acta Trop. 2003; 89:73–84. PMID: 14636985.
Article24. Pritchard DI, Quinnell RJ, McKean PG, Walsh L, Leggett KV, Slater AF, Raiko A, Dale DD, Keymer AE. Antigenic cross-reactivity between Necator americanus and Ascaris lumbricoides in a community in Papua New Guinea infected predominantly with hookworm. Trans R Soc Trop Med Hyg. 1991; 85:511–514. PMID: 1755061.
Article25. Slater JE. Characterization of allergen extracts. Dev Biol (Basel). 2005; 122:145–152. PMID: 16375259.26. Winter JA, Davies OR, Brown AP, Garnett MC, Stolnik S, Pritchard D. The assessment of hookworm calreticulin as a potential vaccine for necatoriasis. Parasite Immunol. 2005; 27:139–146. PMID: 15910422.
Article27. Nara T, Iizumi K, Ohmae H, Sy OS, Tsubota S, Inaba Y, Tsubouchi A, Tanabe M, Kojima S, Aoki T. Antibody isotype responses to paramyosin, a vaccine candidate for schistosomiasis, and their correlations with resistance and fibrosis in patients infected with Schistosoma japonicum in Leyte, The Philippines. Am J Trop Med Hyg. 2007; 76:384–391. PMID: 17297052.
Article28. Pascual CY, Crespo JF, San Martin S, Ornia N, Ortega N, Caballero T, Muñoz-Pereira M, Martin-Esteban M. Cross-reactivity between IgE-binding proteins from Anisakis, German cockroach, and chironomids. Allergy. 1997; 52:514–520. PMID: 9201362.
Article29. Hooper SL, Thuma JB. Invertebrate muscles: muscle specific genes and proteins. Physiol Rev. 2005; 85:1001–1060. PMID: 15987801.
Article30. Pérez-Pérez J, Fernández-Caldas E, Marañón F, Sastre J, Bernal ML, Rodríguez J, Bedate CA. Molecular cloning of paramyosin, a new allergen of Anisakis simplex. Int Arch Allergy Immunol. 2000; 123:120–129. PMID: 11060483.31. Palmer LJ, Celedón JC, Weiss ST, Wang B, Fang Z, Xu X. Ascaris lumbricoides infection is associated with increased risk of childhood asthma and atopy in rural China. Am J Respir Crit Care Med. 2002; 165:1489–1493. PMID: 12045121.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analysis of Simultaneous Positivity to Multiple Allergens on MAST CLA Test
- Food and house dust mite allergens in children with atopic dermatitis
- A Comparative Study of Atopy Patch Test Using House Dust Mite Antigens with Skin Prick Test and Specific Serum IgE Level in Atopic Dermatitis
- Patch test and Specific IgE Concentration with House Dust Mite Antigens in Atopic Dermatitis Patients
- Type I Allergy to House Dust Mite and Familial BACKGROUND of Respiratory Atopy in Patients with Atopic Dermatitis