Asia Pac Allergy.
2013 Jan;3(1):35-41. 10.5415/apallergy.2013.3.1.35.
Milk allergy in the neonatal intensive care unit: comparison between premature and full-term neonates
- Affiliations
-
- 1Department of Pediatrics, Graduate School of Medicine, Chiba University, Chiba 260-8670, Japan. m0ritan@chiba-u.jp
- 2Department of Neonatology, Chiba Kaihin Municipal Hospital, Chiba 261-0012, Japan.
- KMID: 2397333
- DOI: http://doi.org/10.5415/apallergy.2013.3.1.35
Abstract
- BACKGROUND
There have been several reports on neonates with milk allergy in a neonatal ward. This type of allergy is mostly categorized as a non-IgE-mediated food allergy. Although most cases of milk allergy occur in the first few months of life, the differences in clinical characteristics between premature and full-term neonates are still unclear.
OBJECTIVE
This study aimed to clarify the differences in clinical characteristics of milk allergy between premature and full-term neonates.
METHODS
We retrospectively evaluated 2,116 neonates admitted to the Department of Neonatology, Chiba Kaihin Municipal Hospital, between 2001 and 2007.
RESULTS
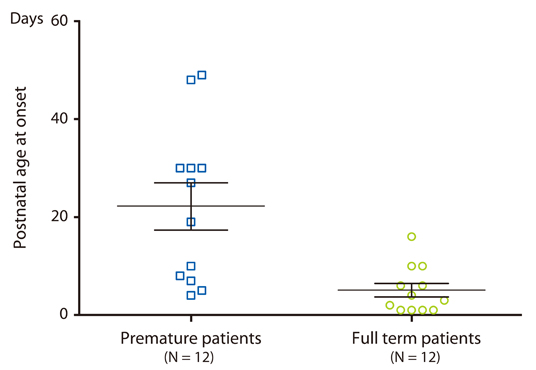
We identified 24 neonates strongly suspected of having milk allergy because of symptoms, such as bloody stools, repeated vomiting, diminished sucking and abdominal distension, as well as objective laboratory findings of eosinophilia in stool cytology and/or positive results for a rectal milk challenge test. Twelve of these 24 neonates were premature (median gestational age, 31 ± 3 weeks; median birth weight, 1,656 ± 592 g) and the other 12 were full-term (median gestational age, 38 ± 1 weeks; median birth weight, 2,760 ± 560 g). There were no differences in symptoms and time to start of feeding between premature and full-term neonates, but there was a significant difference in the median postnatal age at onset (premature neonates: 23 days; vs. full-term neonates: 3.5 days; p < 0.01).
CONCLUSION
All premature neonates developed a milk allergy after 32 weeks of corrected gestational age, suggesting that the development of milk allergy requires a certain degree of immunological maturation.
Keyword
MeSH Terms
Figure
Reference
-
1. Boné J, Claver A, Guallar I, Plaza AM. Allergic proctocolitis, food-induced enterocolitis: immune mechanisms, diagnosis and treatment. Allergol Immunopathol (Madr). 2009. 37:36–42.
Article2. Gryboski JD, Burkle F, Hillman R. Milk induced colitis in an infant. Pediatrics. 1966. 38:299–302.
Article3. Gryboski JD. Gastrointestinal milk allergy in infants. Pediatrics. 1967. 40:354–362.
Article4. Powell GK. Enterocolitis in low-birth-weight infants associated with milk and soy protein intolerance. J Pediatr. 1976. 88:840–844.
Article5. Powell GK. Food protein-induced enterocolitis of infancy: differential diagnosis and management. Compr Ther. 1986. 12:28–37.6. Sicherer SH, Eigenmann PA, Sampson HA. Clinical features of food protein-induced enterocolitis syndrome. J Pediatr. 1998. 133:214–219.
Article7. Nowak-Wegrzyn A, Muraro A. Food protein-induced enterocolitis syndrome. Curr Opin Allergy Clin Immunol. 2009. 9:371–377.8. Nowak-Wegrzyn A. Metcalfe DD, Sampson HA, Simon RA, editors. Food protein-induced enterocolitis and enteropathies. Food allergy: adverse reactions to foods and food additives. 2008. Boston: Blackwell Scientific Publications;195–210.
Article9. Nomura I, Morita H, Hosokawa S, Hoshina H, Fukuie T, Watanabe M, Ohtsuka Y, Shoda T, Terada A, Takamasu T, Arai K, Ito Y, Ohya Y, Saito H, Matsumoto K. Four distinct subtypes of non-IgE-mediated gastrointestinal food allergies in neonates and infants, distinguished by their initial symptoms. J Allergy Clin Immunol. 2011. 127:685–688.e1-8.
Article10. Powell GK. Milk- and soy-induced enterocolitis of infancy. Clinical features and standardization of challenge. J Pediatr. 1978. 93:553–560.11. Sicherer SH. Food protein-induced enterocolitis syndrome: case presentations and management lessons. J Allergy Clin Immunol. 2005. 115:149–156.
Article12. Hwang JB, Lee SH, Kang YN, Kim SP, Suh SI, Kam S. Indexes of suspicion of typical cow's milk protein-induced enterocolitis. J Korean Med Sci. 2007. 22:993–997.
Article13. Kawai M, Kubota A, Ida S, Yamamura Y, Yoshimura N, Takeuchi M, Nakayama M, Okuyama H, Oue T, Kawahara H, Okada A. Cow's milk allergy presenting Hirschsprung's disease-mimicking symptoms. Pediatr Surg Int. 2005. 21:850–852.
Article14. Miyazawa T, Itahashi K, Imai T. Management of neonatal cow's milk allergy in high-risk neonates. Pediatr Int. 2009. 51:544–547.
Article15. Mehr S, Kakakios A, Frith K, Kemp AS. Food protein-induced enterocolitis syndrome: 16-year experience. Pediatrics. 2009. 123:e459–e464.
Article16. Honma K, Kohno Y, Hirano K, Shimojo N, Suzuki H, Hoshioka A, Niimi H. Diagnosis of food allergy based on rectal mucosal cytology. Arerugi. 1992. 41:749–756.17. Faber MR, Rieu P, Semmekrot BA, Van Krieken JH, Tolboom JJ, Draaisma JM. Allergic colitis presenting within the first hours of premature life. Acta Paediatr. 2005. 94:1514–1515.
Article18. Jones AC, Miles EA, Warner JO, Colwell BM, Bryant TN, Warner JA. Fetal peripheral blood mononuclear cell proliferative responses to mitogenic and allergenic stimuli during gestation. Pediatr Allergy Immunol. 1996. 7:109–116.
Article19. Warner JA, Jones CA, Jones AC, Warner JO. Prenatal origins of allergic disease. J Allergy Clin Immunol. 2000. 105:S493–S498.
Article20. Chung HL, Hwang JB, Park JJ, Kim SG. Expression of transforming growth factor beta1, transforming growth factor type I and II receptors, and TNF-alpha in the mucosa of the small intestine in infants with food protein-induced enterocolitis syndrome. J Allergy Clin Immunol. 2002. 109:150–154.21. Dupont C, Soulaines P, Lapillonne A, Donne N, Kalach N, Benhamou P. Atopy patch test for early diagnosis of cow's milk allergy in preterm infants. J Pediatr Gastroenterol Nutr. 2010. 50:463–464.
Article22. Srinivasan P, Brandler M, D'Souza A, Millman P, Moreau H. Allergic enterocolitis presenting as recurrent necrotizing enterocolitis in preterm neonates. J Perinatol. 2010. 30:431–433.
Article23. Gordon PV, Clark R. In response to the case report of allergic enterocolitis in a preterm neonate: how prevalent is systemic eosinophilia with NEC? J Perinatol. 2011. 31:297–298.
Article24. Wilson NW, Self TW, Hamburger RN. Severe cow's milk induced colitis in an exclusively breast-fed neonate. Case report and clinical review of cow's milk allergy. Clin Pediatr (Phila). 1990. 29:77–80.25. Monti G, Castagno E, Liguori SA, Lupica MM, Tarasco V, Viola S, Tovo PA. Food protein-induced enterocolitis syndrome by cow's milk proteins passed through breast milk. J Allergy Clin Immunol. 2011. 127:679–680.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Birth Statistics and Mortality Rates for Neonatal Intensive Care Units in Korea during 2007: Collective Results from 57 Hospitals
- Evaluation of Preterm Delivery between 32(+0)-33(+6) Weeks of Gestation
- Neonatal hypertension: concerns within and beyond the neonatal intensive care unit
- Organisation of Special and Intensive Care Facilities for Babies
- Periventricular-Intraventricular Hemorrhage in the Full-term Infant