Asia Pac Allergy.
2015 Jul;5(3):163-169. 10.5415/apallergy.2015.5.3.163.
Effect of high-dose sublingual immunotherapy on respiratory infections in children allergic to house dust mite
- Affiliations
-
- 1Department of Pediatrics, San Paolo Hospital, 20142 Milan, Italy.
- 2Department of Medicine, IRCCS-Azienda Ospedaliera Universitaria San Martino, 16132 Genoa, Italy. gio.cip@libero.it
- 3Allergy/Pulmonary Rehabilitation, ICP Hospital, 20100 Milan, Italy.
- 4Medical and Scientific Department, Stallergenes Italy, 20155 Milan, Italy.
- KMID: 2397046
- DOI: http://doi.org/10.5415/apallergy.2015.5.3.163
Abstract
- BACKGROUND
Allergic rhinitis is characterized by eosinophil inflammation. Allergic inflammation may induce susceptibility to respiratory infections (RI). House dust mite (HDM) sensitization is very frequent in childhood. Allergen immunotherapy may cure allergy as it restores a physiologic immune and clinical tolerance to allergen and exerts anti-inflammatory activity.
OBJECTIVE
This study investigated whether six-month high-dose, such as 300 IR (index of reactivity), HDM-sublingual immunotherapy (SLIT) could affect RI in allergic children.
METHODS
Globally, 40 HDM allergic children (18 males; mean age, 9.3 years) were subdivided in 2 groups: 20 treated by symptomatic drugs (group 1) and 20 by high-dose HDM-SLIT (group 2), since September 2012 to April 2013. The daily maintenance dose of HDM-SLIT was 4 pressures corresponding to 24, 4.8, and 60 µg, respectively of the major allergens Dermatophagoides pteronyssinus (Der p) 1, Der p 2, and Dermatophagoides farinae (Der f) 1. RI was diagnosed when at least 2 symptoms or signs, and fever were present for at least 48 hours. A family pediatrician provided diagnosis on a clinical ground.
RESULTS
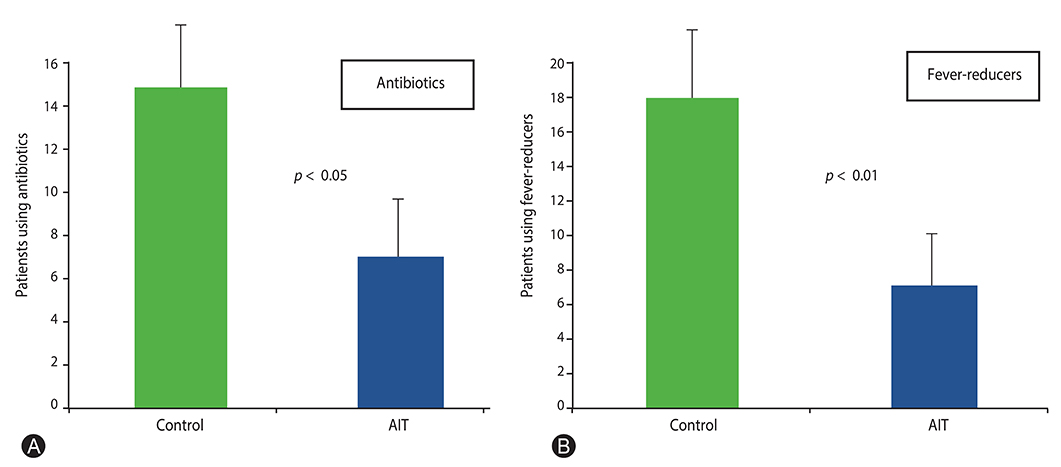
SLIT-treated children had significantly (p = 0.01) less RI episodes (3.5) than control group (5.45). About secondary outcomes, SLIT-treated children had less episodes of pharyngo-tonsillitis (p < 0.05) and bronchitis (p < 0.005), and snoring (p < 0.05) than control group. In addition, SLIT-treated children had less fever (p < 0.01) and took fewer medications, such as antibiotics (p < 0.05) and fever-reducers (p < 0.01), than control group.
CONCLUSION
This preliminary study might suggest that also a short course (6 months) of high-dose SLIT, titrated in µg of major allergens, could reduce RI in allergic children.
MeSH Terms
-
Allergens
Anti-Bacterial Agents
Bronchitis
Child*
Dermatophagoides farinae
Dermatophagoides pteronyssinus
Desensitization, Immunologic
Diagnosis
Dust*
Eosinophils
Fever
Humans
Hypersensitivity
Immunotherapy
Inflammation
Male
Mites
Pyroglyphidae*
Respiratory Tract Infections*
Rhinitis, Allergic
Snoring
Sublingual Immunotherapy*
Allergens
Anti-Bacterial Agents
Dust
Figure
Cited by 1 articles
-
The sticky relationship between allergies and infections
Jiu-Yao Wang
Asia Pac Allergy. 2015;5(3):133-135. doi: 10.5415/apallergy.2015.5.3.133.
Reference
-
1. Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, Zuberbier T, Baena-Cagnani CE, Canonica GW, van Weel C, Agache I, Aït-Khaled N, Bachert C, Blaiss MS, Bonini S, Boulet LP, Bousquet PJ, Camargos P, Carlsen KH, Chen Y, Custovic A, Dahl R, Demoly P, Douagui H, Durham SR, van Wijk RG, Kalayci O, Kaliner MA, Kim YY, Kowalski ML, Kuna P, Le LT, Lemiere C, Li J, Lockey RF, Mavale-Manuel S, Meltzer EO, Mohammad Y, Mullol J, Naclerio R, O'Hehir RE, Ohta K, Ouedraogo S, Palkonen S, Papadopoulos N, Passalacqua G, Pawankar R, Popov TA, Rabe KF, Rosado-Pinto J, Scadding GK, Simons FE, Toskala E, Valovirta E, van Cauwenberge P, Wang DY, Wickman M, Yawn BP, Yorgancioglu A, Yusuf OM, Zar H, Annesi-Maesano I, Bateman ED, Ben Kheder A, Boakye DA, Bouchard J, Burney P, Busse WW, Chan-Yeung M, Chavannes NH, Chuchalin A, Dolen WK, Emuzyte R, Grouse L, Humbert M, Jackson C, Johnston SL, Keith PK, Kemp JP, Klossek JM, Larenas-Linnemann D, Lipworth B, Malo JL, Marshall GD, Naspitz C, Nekam K, Niggemann B, Nizankowska-Mogilnicka E, Okamoto Y, Orru MP, Potter P, Price D, Stoloff SW, Vandenplas O, Viegi G, Williams D. World Health Organization. GA(2)LEN. AllerGen. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy. 2008; 63:Suppl 86. 8–160.2. Miraglia Del Giudice M, Marseglia A, Leonardi S, La Rosa M, Salpietro C, Brunese FP, Arrigo T, Perrone L. Allergic rhinitis and quality of life in children. Int J Immunopathol Pharmacol. 2011; 24:4 Suppl. 25–28.
Article3. Ciprandi G, Tosca MA, Fasce L. Allergic children have more numerous and severe respiratory infections than non-allergic children. Pediatr Allergy Immunol. 2006; 17:389–391.
Article4. Fenoglio D, Ferrera A, Ferrera F, Sormani MP, Di Gioacchino M, Ciprandi G. Patients with allergic rhinitis show an allergen-specific interferon-gamma defect. Eur J Inflamm. 2008; 6:87–91.
Article5. Ciprandi G, Buscaglia S, Pesce G, Pronzato C, Ricca V, Parmiani S, Bagnasco M, Canonica GW. Minimal persistent inflammation is present at mucosal level in patients with asymptomatic rhinitis and mite allergy. J Allergy Clin Immunol. 1995; 96(6 Pt 1):971–979.
Article6. Gelardi M, Peroni DG, Incorvaia C, Quaranta N, De Luca C, Barberi S, Dell'albani I, Landi M, Frati F, de Beaumont O. Seasonal changes in nasal cytology in mite-allergic patients. J Inflamm Res. 2014; 7:39–44.7. Yilmaz O, Bakirtas A, Ertoy Karagol HI, Topal E, Demirsoy MS. Allergic rhinitis may impact the recovery of pulmonary function tests after moderate/severe asthma exacerbation in children. Allergy. 2014; 69:652–657.
Article8. Szefler SJ. Advances in pediatric asthma in 2013: coordinating asthma care. J Allergy Clin Immunol. 2014; 133:654–661.
Article9. Tantilipikorn P. The relationship between allergic rhinitis and viral infections. Curr Opin Otolaryngol Head Neck Surg. 2014; 22:249–252.
Article10. Linkov G, Toskala E. Sublingual immunotherapy: what we can learn from the European experience. Curr Opin Otolaryngol Head Neck Surg. 2014; 22:208–210.11. Ciprandi G, Fenoglio D, Ferrera F, De Amici M, Marseglia G. ELISPOT and ELISA assessment of interferon-gamma after sublingual immunotherapy. Eur J Inflamm. 2010; 8:31–35.
Article12. Ciprandi G, Incorvaia C, Dell'Albani I, Di Cara G, Barberi S, Puccinelli P, Frati F. RINOBIT Study Group. Allergen immunotherapy may exert an extra-anti-allergic activity in children. J Biol Regul Homeost Agents. 2013; 27:1053–1057.13. Canonica GW, Cox L, Pawankar R, Baena-Cagnani CE, Blaiss M, Bonini S, Bousquet J, Calderón M, Compalati E, Durham SR, van Wijk RG, Larenas-Linnemann D, Nelson H, Passalacqua G, Pfaar O, Rosário N, Ryan D, Rosenwasser L, Schmid-Grendelmeier P, Senna G, Valovirta E, Van Bever H, Vichyanond P, Wahn U, Yusuf O. Sublingual immunotherapy: World Allergy Organization position paper 2013 update. World Allergy Organ J. 2014; 7:6.
Article14. Michaud B, Gouvis-Echraghi R, Candon S, Couderc R, Jais JP, Bach JF, Chatenoud L, Just J. Quantification of circulating house dust mite-specific IL-4- and IL-13-secreting T cells correlates with rhinitis severity in asthmatic children and varies with the seasons. Clin Exp Allergy. 2014; 44:222–230.
Article15. Varricchio A, Capasso M, Di Gioacchino M, Ciprandi G. Inhaled thiamphenicol and acetylcysteine in children with acute bacterial rhinopharyngitis. Int J Immunopathol Pharmacol. 2008; 21:625–629.16. Cirillo I, Marseglia G, Klersy C, Ciprandi G. Allergic patients have more numerous and prolonged respiratory infections than nonallergic subjects. Allergy. 2007; 62:1087–1090.
Article17. Bardin PG, Fraenkel DJ, Sanderson G, Dorward M, Lau LC, Johnston SL, Holgate ST. Amplified rhinovirus colds in atopic subjects. Clin Exp Allergy. 1994; 24:457–464.
Article18. Corne JM, Marshall C, Smith S, Schreiber J, Sanderson G, Holgate ST, Johnston SL. Frequency, severity, and duration of rhinovirus infections in asthmatic and non-asthmatic individuals: a longitudinal cohort study. Lancet. 2002; 359:831–834.
Article19. Kusel MM, Kebadze T, Johnston SL, Holt PG, Sly PD. Febrile respiratory illnesses in infancy and atopy are risk factors for persistent asthma and wheeze. Eur Respir J. 2012; 39:876–882.
Article20. Bernstein JA. Characterizing rhinitis subtypes. Am J Rhinol Allergy. 2013; 27:457–460.
Article21. Kim JH, Moon BJ, Gong CH, Kim NH, Jang YJ. Detection of respiratory viruses in adult patients with perennial allergic rhinitis. Ann Allergy Asthma Immunol. 2013; 111:508–511.
Article22. Santillan Salas CF, Mehra S, Pardo Crespo MR, Juhn YJ. Atopic conditions other than asthma and risk of the 2009 novel H1N1 infection in children: a case-control study. Allergy Asthma Proc. 2013; 34:459–466.
Article23. Rantala A, Jaakkola JJ, Jaakkola MS. Respiratory infections in adults with atopic disease and IgE antibodies to common aeroallergens. PLoS One. 2013; 8:e68582.
Article24. del Giudice MM, Leonardi S, Ciprandi G, Galdo F, Gubitosi A, La Rosa M, Salpietro C, Marseglia G, Perrone L. Probiotics in childhood: allergic illness and respiratory infections. J Clin Gastroenterol. 2012; 46:S69–S72.25. Sedaghat AR, Gray ST, Wilke CO, Caradonna DS. Risk factors for development of chronic rhinosinusitis in patients with allergic rhinitis. Int Forum Allergy Rhinol. 2012; 2:370–375.
Article26. Veling MC. The role of allergy in pediatric rhinosinusitis. Curr Opin Otolaryngol Head Neck Surg. 2013; 21:271–276.
Article27. Cosmi L, Santarlasci V, Angeli R, Liotta F, Maggi L, Frosali F, Rossi O, Falagiani P, Riva G, Romagnani S, Annunziato F, Maggi E. Sublingual immunotherapy with Dermatophagoides monomeric allergoid down-regulates allergen-specific immunoglobulin E and increases both interferon-gamma- and interleukin-10-production. Clin Exp Allergy. 2006; 36:261–272.
Article28. Ciprandi G, Continia P, Fenoglio D, Sormani MP, Negrini S, Puppo F, Indiveri F. Relationship between soluble HLA-G and HLA-A,-B,-C serum levels, and interferon-gamma production after sublingual immunotherapy in patients with allergic rhinitis. Hum Immunol. 2008; 69:409–413.29. Cox L. Sublingual immunotherapy for aeroallergens: status in the United States. Allergy Asthma Proc. 2014; 35:34–42.
Article30. Pajno GB, Caminiti L, Passalacqua G. Changing the route of immunotherapy administration: an 18-year survey in pediatric patients with allergic rhinitis and asthma. Allergy Asthma Proc. 2013; 34:523–526.
Article31. Busse WW, Morgan WJ, Gergen PJ, Mitchell HE, Gern JE, Liu AH, Gruchalla RS, Kattan M, Teach SJ, Pongracic JA, Chmiel JF, Steinbach SF, Calatroni A, Togias A, Thompson KM, Szefler SJ, Sorkness CA. Randomized trial of omalizumab (anti-IgE) for asthma in inner-city children. N Engl J Med. 2011; 364:1005–1015.
Article32. Darveaux JI, Lemanske RF Jr. Infection-related asthma. J Allergy Clin Immunol Pract. 2014; 2:658–663.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Asthma Management Educational Program on The Disease Related Knowledge, Stress, and Self-efficacy of Asthmatics Allergic to House Dust Mite
- Effect on quality of life of the mixed house dust mite/weed pollen extract immunotherapy
- Comparison of subcutaneous and sublingual immunotherapy in house dust mite-induced allergic rhinitis
- The effect of house dust mite conventional immunotherapy on the production of IL-4 and interferon-gamma from the peripheral blood T cells in asthmatic children
- The Effect of Specific Immunotherapy with House Dust Mite AIleI-gen in ChiIdhood Asthma