Restor Dent Endod.
2015 Aug;40(3):209-215. 10.5395/rde.2015.40.3.209.
Do conventional glass ionomer cements release more fluoride than resin-modified glass ionomer cements?
- Affiliations
-
- 1School of Dentistry, Federal University of Amazonas, Manaus, Brazil.
- 2Postgraduate Program in Dentistry, School of Dentistry, Federal University of Amazonas, Manaus, Brazil. flaviacohencarneiro@gmail.com
- 3School of Health Sciences, State University of Amazonas, Manaus, Brazil.
- KMID: 2396464
- DOI: http://doi.org/10.5395/rde.2015.40.3.209
Abstract
OBJECTIVES
The aim of this study was to evaluate the fluoride release of conventional glass ionomer cements (GICs) and resin-modified GICs.
MATERIALS AND METHODS
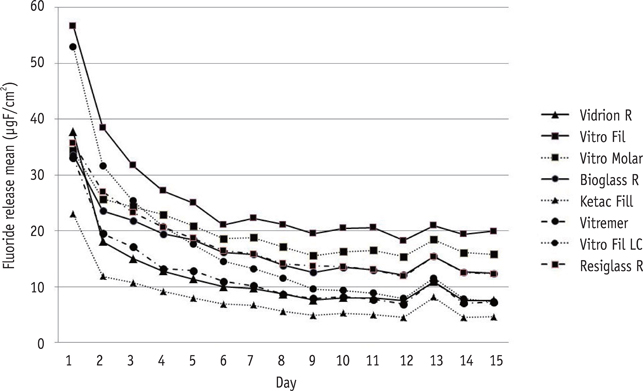
The cements were grouped as follows: G1 (Vidrion R, SS White), G2 (Vitro Fil, DFL), G3 (Vitro Molar, DFL), G4 (Bioglass R, Biodinamica), and G5 (Ketac Fil, 3M ESPE), as conventional GICs, and G6 (Vitremer, 3M ESPE), G7 (Vitro Fil LC, DFL), and G8 (Resiglass, Biodinamica) as resin-modified GICs. Six specimens (8.60 mm in diameter; 1.65 mm in thickness) of each material were prepared using a stainless steel mold. The specimens were immersed in a demineralizing solution (pH 4.3) for 6 hr and a remineralizing solution (pH 7.0) for 18 hr a day. The fluoride ions were measured for 15 days. Analysis of variance (ANOVA) and Tukey's test with 5% significance were applied.
RESULTS
The highest amounts of fluoride release were found during the first 24 hr for all cements, decreasing abruptly on day 2, and reaching gradually decreasing levels on day 7. Based on these results, the decreasing scale of fluoride release was as follows: G2 > G3 > G8 = G4 = G7 > G6 = G1 > G5 (p < 0.05).
CONCLUSIONS
There were wide variations among the materials in terms of the cumulative amount of fluoride ion released, and the amount of fluoride release could not be attributed to the category of cement, that is, conventional GICs or resin-modified GICs.
Keyword
MeSH Terms
Figure
Reference
-
1. Mungara J, Philip J, Joseph E, Rajendran S, Elangovan A, Selvaraju G. Comparative evaluation of fluoride release and recharge of pre-reacted glass ionomer composite and nano-ionomeric glass ionomer with daily fluoride exposure: an in vitro study. J Indian Soc Pedod Prev Dent. 2013; 31:234–239.
Article2. Upadhyay S, Rao A, Shenoy R. Comparison of the amount of fluoride release from nanofilled resin modified glass ionomer, conventional and resin modified glass ionomer cements. J Dent (Tehran). 2013; 10:134–140.3. Markovic DL, Petrovic BB, Peric TO. Fluoride content and recharge ability of five glass ionomer dental materials. BMC Oral Health. 2008; 8:21.
Article4. Wilson AD, Kent BE. A new translucent cement for dentistry. The glass ionomer cement. Br Dent J. 1972; 132:133–135.
Article5. Neelakantan P, John S, Anand S, Sureshbabu N, Subbarao C. Fluoride release from a new glass-ionomer cement. Oper Dent. 2011; 36:80–85.
Article6. Shiozawa M, Takahashi H, Iwasaki N. Fluoride release and mechanical properties after 1-year water storage of recent restorative glass ionomer cements. Clin Oral Investig. 2014; 18:1053–1060.
Article7. McLean JW, Wilson AD. The clinical development of glass-ionomer cements III. The erosion lesion. Aust Dent J. 1977; 22:190–195.8. Levallois B, Fovet Y, Lapeyre L, Gal JY. In vitro fluoride release from restorative materials in water versus artificial saliva medium (SAGF). Dent Mater. 1998; 14:441–447.
Article9. Tiwari S, Nandlal B. Comparative evaluation of fluoride release from hydroxyapatite incorporated and conventional glass ionomer cement: an in vitro study. J Indian Soc Pedod Prev Dent. 2012; 30:284–287.
Article10. Qvist V, Manscher E, Teglers PT. Resin-modified and conventional glass ionomer restorations in primary teeth: 8-year results. J Dent. 2004; 32:285–294.
Article11. Qvist V, Poulsen A, Teglers PT, Mjör IA. Fluorides leaching from restorative materials and the effect on adjacent teeth. Int Dent J. 2010; 60:156–160.12. Hattab FN, Amin WM. Fluoride release from glass ionomer restorative materials and the effects of surface coating. Biomaterials. 2001; 22:1449–1458.
Article13. Diaz-Arnold AM, Holmes DC, Wistrom DW, Swift EJ Jr. Short-term fluoride release/uptake of glass ionomer restoratives. Dent Mater. 1995; 11:96–101.
Article14. Dionysopoulos D, Koliniotou-Koumpia E, Helvatzoglou-Antoniades M, Kotsanos N. Fluoride release and recharge abilities of contemporary fluoride-containing restorative materials and dental adhesives. Dent Mater J. 2013; 32:296–304.
Article15. Wiegand A, Buchalla W, Attin T. Review on fluoride-releasing restorative materials--Fluoride release and uptake characteristics, antibacterial activity and influence on caries formation. Dent Mater. 2007; 23:343–362.
Article16. Billington RW, Williams JA, Dorban A, Pearson GJ. Glass ionomer cement: evidence pointing to fluorine release in the form of monofluorophosphate in addition to fluoride ion. Biomaterials. 2004; 25:3399–3402.
Article17. Can-Karabulut DC, Batmaz I, Solak H, Taştekin M. Linear regression modeling to compare fluoride release profiles of various restorative materials. Dent Mater. 2007; 23:1057–1065.
Article18. Bahadure RN, Pandey RK, Kumar R, Gopal K, Singh RK. An estimation of fluoride release from various dental restorative materials at different pH: in vitro study. J Indian Soc Pedod Prev Dent. 2012; 30:122–126.
Article19. Moreau JL, Xu HH. Fluoride releasing restorative materials: effects of pH on mechanical properties and ion release. Dent Mater. 2010; 26:e227–e235.
Article20. Jeong YN, Yang SY, Park BJ, Park YJ, Hwang YC, Hwang IN, Oh WM. Physical and chemical properties of experimental mixture of mineral trioxide aggregate and glass ionomer cement. J Korean Acad Conserv Dent. 2010; 35:344–352.
Article21. Seppä L, Forss H, Ogaard B. The effect of fluoride application on fluoride release and the antibacterial action of glass ionomers. J Dent Res. 1993; 72:1310–1314.
Article22. Grobler SR, Rossouw RJ, Van Wyk Kotze TJ. A comparison of fluoride release from various dental materials. J Dent. 1998; 26:259–265.
Article23. Carvalho AS, Cury JA. Fluoride release from some dental materials in different solutions. Oper Dent. 1999; 24:14–19.24. Williams JA, Billington RW, Pearson GJ. The influence of sample dimensions on fluoride ion release from a glass ionomer restorative cement. Biomaterials. 1999; 20:1327–1337.
Article25. Dionysopoulos P, Kotsanos N, Pataridou A. Fluoride release and uptake by four new fluoride releasing materials. J Oral Rehabil. 2003; 30:866–872.26. Xu X, Burgess JO. Compressive strength, fluoride release and recharge of fluoride-releasing materials. Biomaterials. 2003; 24:2451–2461.
Article27. Hayacibara MF, Ambrosano GM, Cury JA. Simultaneous release of fluoride and aluminum from dental materials in various immersion media. Oper Dent. 2004; 29:16–22.28. Hsu HM, Huang GF, Chang HH, Wang YL, Guo MK. A continuous flow system for assessing fluoride release/uptake of fluoride-containing restorative materials. Dent Mater. 2004; 20:740–749.
Article29. Chan WD, Yang L, Wan W, Rizkalla AS. Fluoride release from dental cements and composites: a mechanistic study. Dent Mater. 2006; 22:366–373.
Article30. Gandolfi MG, Chersoni S, Acquaviva GL, Piana G, Prati C, Mongiorgi R. Fluoride release and absorption at different pH from glass-ionomer cements. Dent Mater. 2006; 22:441–449.
Article31. Selimović-Dragaš M, Hasić-Branković L, Korać F, Ðapo N, Huseinbegović A, Kobašlija S, Lekić M, Hatibović-Kofman Š. In vitro fluoride release from a different kind of conventional and resin modified glass-ionomer cements. Bosn J Basic Med Sci. 2013; 13:197–202.
Article32. Mousavinasab SM, Meyers I. Fluoride release by glass ionomer cements, compomer and giomer. Dent Res J (Isfahan). 2009; 6:75–81.33. McKenzie MA, Linden RW, Nicholson JW. The physical properties of conventional and resin-modified glass-ionomer dental cements stored in saliva, proprietary acidic beverages, saline and water. Biomaterials. 2003; 24:4063–4069.
Article34. Rothwell M, Anstice HM, Pearson GJ. The uptake and release of fluoride by ion-leaching cements after exposure to toothpaste. J Dent. 1998; 26:591–597.
Article35. Gao W, Smales RJ. Fluoride release/uptake of conventional and resin-modified glass ionomers, and compomers. J Dent. 2001; 29:301–306.
Article36. Rodrigues JA, Marchi GM, Serra MC, Hara AT. Visual evaluation of in vitro cariostatic effect of restorative materials associated with dentifrices. Braz Dent J. 2005; 16:112–118.
Article37. Dijkman GE, de Vries J, Lodding A, Arends J. Long-term fluoride release of visible light-activated composites in vitro: a correlation with in situ demineralization data. Caries Res. 1993; 27:117–123.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- In vitro study on the fluoride release from glass ionomer cements and a fluoride-containing resin
- Fluoride Release and Recharge Properties of Several Fluoride-Containing Restorative Materials
- Effect of Fluoride Recharging on Fluoride Release and Surface Properties of Orthodontic Bracket Adhesives
- Fluoride Release of Several Types of Fluoride-Containing Restorative Materials According to Fluoride Concentration in Toothpaste
- The shear bond strength of two adhesives bonded to composite resin and glass ionomer cement restorations