Restor Dent Endod.
2015 Aug;40(3):202-208. 10.5395/rde.2015.40.3.202.
Effects of solvent volatilization time on the bond strength of etch-and-rinse adhesive to dentin using conventional or deproteinization bonding techniques
- Affiliations
-
- 1School of Dentistry, Federal University of Sergipe, Aracaju, Brazil. fariaesilva.andre@gmail.com
- 2Graduate Program in Dentistry, Federal University of Sergipe, Aracaju, Brazil.
- KMID: 2396463
- DOI: http://doi.org/10.5395/rde.2015.40.3.202
Abstract
OBJECTIVES
This study determined the effect of the air-stream application time and the bonding technique on the dentin bond strength of adhesives with different solvents. Furthermore, the content and volatilization rate of the solvents contained in the adhesives were also evaluated.
MATERIALS AND METHODS
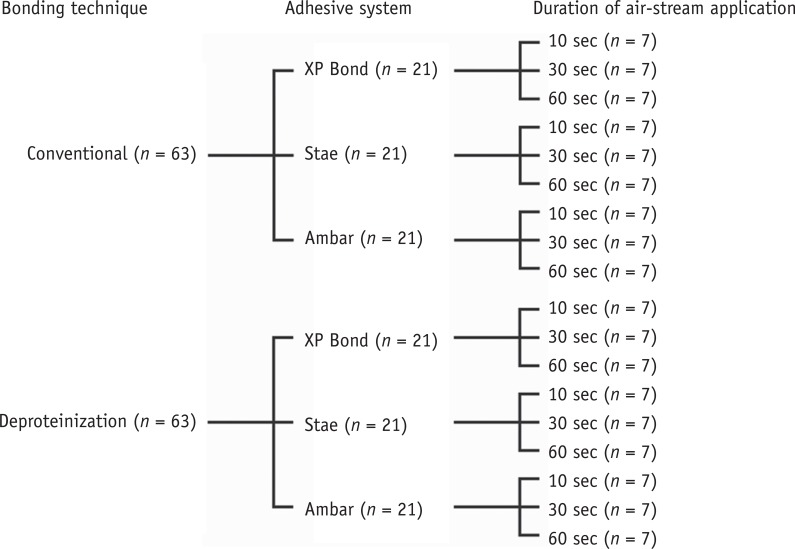
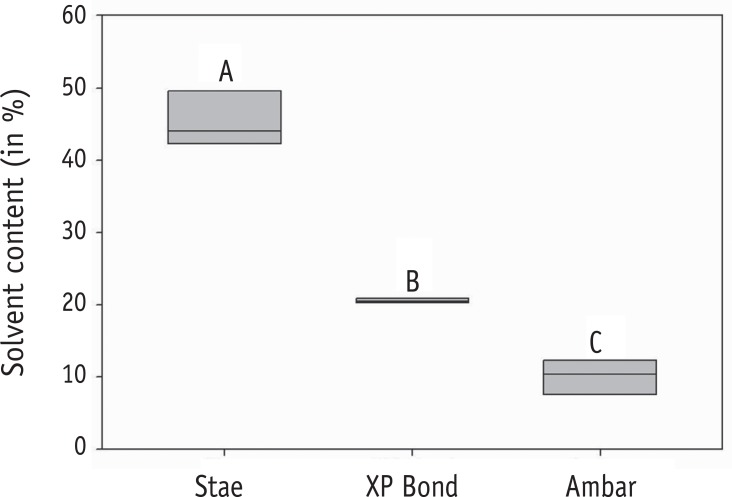
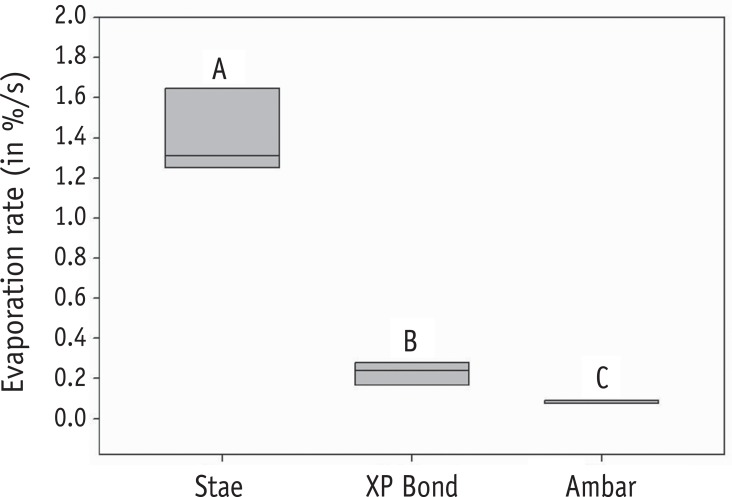
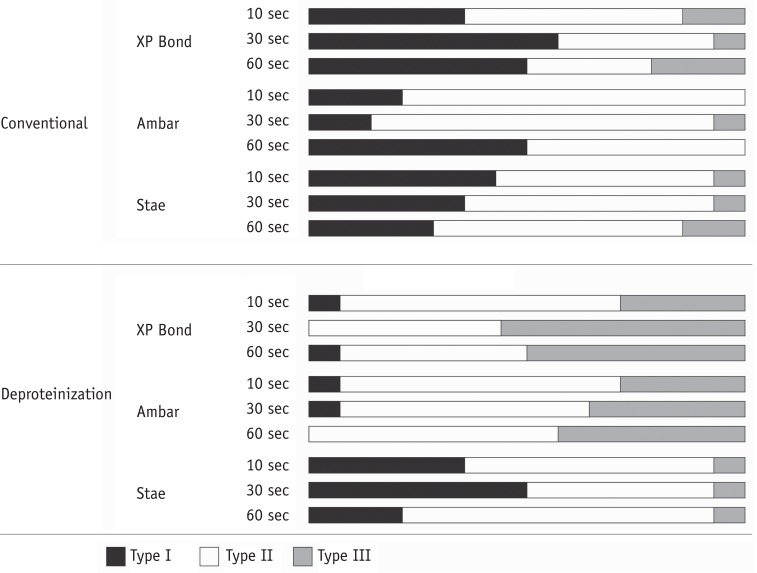
Three adhesive systems with different solvents (Stae, SDI, acetone; XP Bond, Dentsply De Trey, butanol; Ambar, FGM, ethanol) were evaluated. The concentrations and evaporation rates of each adhesive were measured using an analytical balance. After acid-etching and rinsing, medium occlusal dentin surfaces of human molars were kept moist (conventional) or were treated with 10% sodium hypochlorite for deproteinization. After applying adhesives over the dentin, slight air-stream was applied for 10, 30 or 60 sec. Composite cylinders were built up and submitted to shear testing. The data were submitted to ANOVA and Tukey's test (alpha = 0.05).
RESULTS
Stae showed the highest solvent content and Ambar the lowest. Acetone presented the highest evaporation rate, followed by butanol. Shear bond strengths were significantly affected only by the factors of 'adhesive' and 'bonding technique' (p < 0.05), while the factor 'duration of air-stream' was not significant. Deproteinization of dentin increased the bond strength (p < 0.05). Stae showed the lowest bond strength values (p < 0.05), while no significant difference was observed between XP Bond and Ambar.
CONCLUSIONS
Despite the differences in content and evaporation rate of the solvents, the duration of air-stream application did not affect the bond strength to dentin irrespective of the bonding technique.
Keyword
MeSH Terms
Figure
Reference
-
1. Yiu CK, Pashley EL, Hiraishi N, King NM, Goracci C, Ferrari M, Carvalho RM, Pashley DH, Tay FR. Solvent and water retention in dental adhesive blends after evaporation. Biomaterials. 2005; 26:6863–6872. PMID: 15964621.
Article2. Borges FB, Kochhann DE Lima EL, Machado FW, Boscato N, Van De Sande FH, Moraes RR, Cenci MS. Effect of cariogenic challenge on the stability of dentin bonds. J Appl Oral Sci. 2014; 22:68–72. PMID: 24626251.
Article3. Pomacóndor-Hernández C, Antunes AN, Hipólito Vd, Goes MF. Effect of replacing a component of a self-etch adhesive by chlorhexidine on bonding to dentin. Braz Dent J. 2013; 24:335–339. PMID: 24173251.4. Nakabayashi N, Kojima K, Masuhara E. The promotion of adhesion by infiltration of monomers into tooth substrates. J Biomed Mater Res. 1982; 16:265–273. PMID: 7085687.5. Van Meerbeek B, De Munck J, Yoshida Y, Inoue S, Vargas M, Vijav P, Van Landuyt K, Lambrechts P, Vanherle G. Buonocore memorial lecture. Adhesion to enamel and dentin: current status and future challenges. Oper Dent. 2003; 28:215–235. PMID: 12760693.6. Reis A, Loguercio AD, Azevedo CL, de Carvalho RM, da Julio Singer M, Grande RH. Moisture spectrum of demineralized dentin for adhesive systems with different solvent bases. J Adhes Dent. 2003; 5:183–192. PMID: 14621240.7. Cadenaro M, Breschi L, Rueggeberg FA, Suchko M, Grodin E, Agee K, Di Lenarda R, Tay FR, Pashley DH. Effects of residual ethanol on the rate and degree of conversion of five experimental resins. Dent Mater. 2009; 25:621–628. PMID: 19111335.
Article8. Bail M, Malacarne-Zanon J, Silva SM, Anauate-Netto A, Nascimento FD, Amore R, Lewgoy H, Pashley DH, Carrilho MR. Effect of air-drying on the solvent evaporation, degree of conversion and water sorption/solubility of dental adhesive models. J Mater Sci Mater Med. 2012; 23:629–638. PMID: 22210310.
Article9. Emamieh S, Sadr A, Ghasemi A, Torabzadeh H, Akhavanzanjani V, Tagami J. Effects of solvent drying time and water storage on ultimate tensile strength of adhesives. J Investig Clin Dent. 2014; 5:51–57.
Article10. Breschi L, Mazzoni A, Ruggeri A, Cadenaro M, Di Lenarda R, De Stefano Dorigo E. Dental adhesion review: aging and stability of the bonded interface. Dent Mater. 2008; 24:90–101. PMID: 17442386.
Article11. Lima FG, Moraes RR, Demarco FF, Del Pino FA, Powers J. One-bottle adhesives: in vitro analysis of solvent volatilization and sealing ability. Braz Oral Res. 2005; 19:278–283. PMID: 16491256.12. Faria-e-Silva AL, Araújo JE, Rocha GP, de Oliveira Ada S, de Moraes RR. Solvent content and dentin bond strengths using water-wet, ethanol-wet and deproteinization bonding techniques. Acta Odontol Scand. 2013; 71:710–715. PMID: 22900709.
Article13. Reis AF, Oliveira MT, Giannini M, De Goes MF, Rueggeberg FA. The effect of organic solvents on one-bottle adhesives' bond strength to enamel and dentin. Oper Dent. 2003; 28:700–706. PMID: 14653283.14. Abate PF, Rodriguez VI, Macchi RL. Evaporation of solvent in one-bottle adhesives. J Dent. 2000; 28:437–440. PMID: 10856809.
Article15. Nihi FM, Fabre HS, Garcia G, Fernandes KB, Ferreira FB, Wang L. In vitro assessment of solvent evaporation from commercial adhesive systems compared to experimental systems. Braz Dent J. 2009; 20:396–402. PMID: 20126908.16. Silva EM, Duarte PB, Poskus LT, Barcellos AA, Guimarães JG. Nanoleakage and microshear bond strength in deproteinized human dentin. J Biomed Mater Res B Appl Biomater. 2007; 81:336–342. PMID: 17022053.
Article17. Gallo JR, Burgess JO, Xu X. Effect of delayed application on shear bond strength of four fifth-generation bonding systems. Oper Dent. 2001; 26:48–51. PMID: 11203777.18. Oktavian R, Amidelsi V, Rasmito A, Wibawa G. Vapor pressure measurements of ethanol-isooctane and 1-butanol-isooctane systems using a new ebulliometer. Fuel. 2013; 107:47–51.
Article19. Sadr A, Shimada Y, Tagami J. Effects of solvent drying time on micro-shear bond strength and mechanical properties of two self-etching adhesive systems. Dent Mater. 2007; 23:1114–1119. PMID: 17113635.
Article20. Zheng L, Pereira PN, Nakajima M, Sano H, Tagami J. Relationship between adhesive thickness and microtensile bond strength. Oper Dent. 2001; 26:97–104. PMID: 11203783.21. Emamieh S, Sadr A, Ghasemi A, Torabzadeh H, Akhavanzanjani V, Tagami J. Effects of solvent drying time and water storage on ultimate tensile strength of adhesives. J Investig Clin Dent. 2014; 5:51–57.
Article22. Pioch T, Kobaslija S, Schagen B, Götz H. Interfacial micromorphology and tensile bond strength of dentin bonding systems after NaOCl treatment. J Adhes Dent. 1999; 1:135–142. PMID: 11725678.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The effect of saliva decontamination procedures on dentin bond strength after universal adhesive curing
- Do universal adhesives promote bonding to dentin? A systematic review and meta-analysis
- The comparison of microtensile bond strength with immediate and delayed dentin sealing
- Adhesion of 10-MDP containing resin cements to dentin with and without the etch-and-rinse technique
- The effect of various bonding systems on the microtensile bond strength of immediate and delayed dentin sealing