Brain Tumor Res Treat.
2017 Oct;5(2):77-86. 10.14791/btrt.2017.5.2.77.
The Clinical Outcome of Hydroxyurea Chemotherapy after Incomplete Resection of Atypical Meningiomas
- Affiliations
-
- 1Division of Neuro-Oncology and Department of Neurosurgery, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea. yzkim@skku.edu
- KMID: 2396451
- DOI: http://doi.org/10.14791/btrt.2017.5.2.77
Abstract
- BACKGROUND
The aim of this study is to investigate the clinical results of adjuvant chemotherapy with hydroxyurea and to compare those with the results of postoperative radiotherapy after incomplete resection of atypical meningiomas (ATMNGs).
METHODS
We retrospectively reviewed the medical records of 84 patients with ATMNGs diagnosed in the period from January 2000 to December 2014. Clinical data included patient sex and age at the time of surgery, presenting symptoms at diagnosis, location and size of tumor, extent of surgery, use of postoperative radiotherapy or hydroxyurea chemotherapy, duration of follow-up, and progression. In terms of the extent of surgical resection, incomplete resection was defined as Simpson grade II-V.
RESULTS
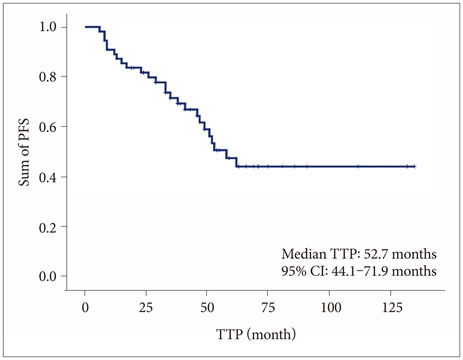
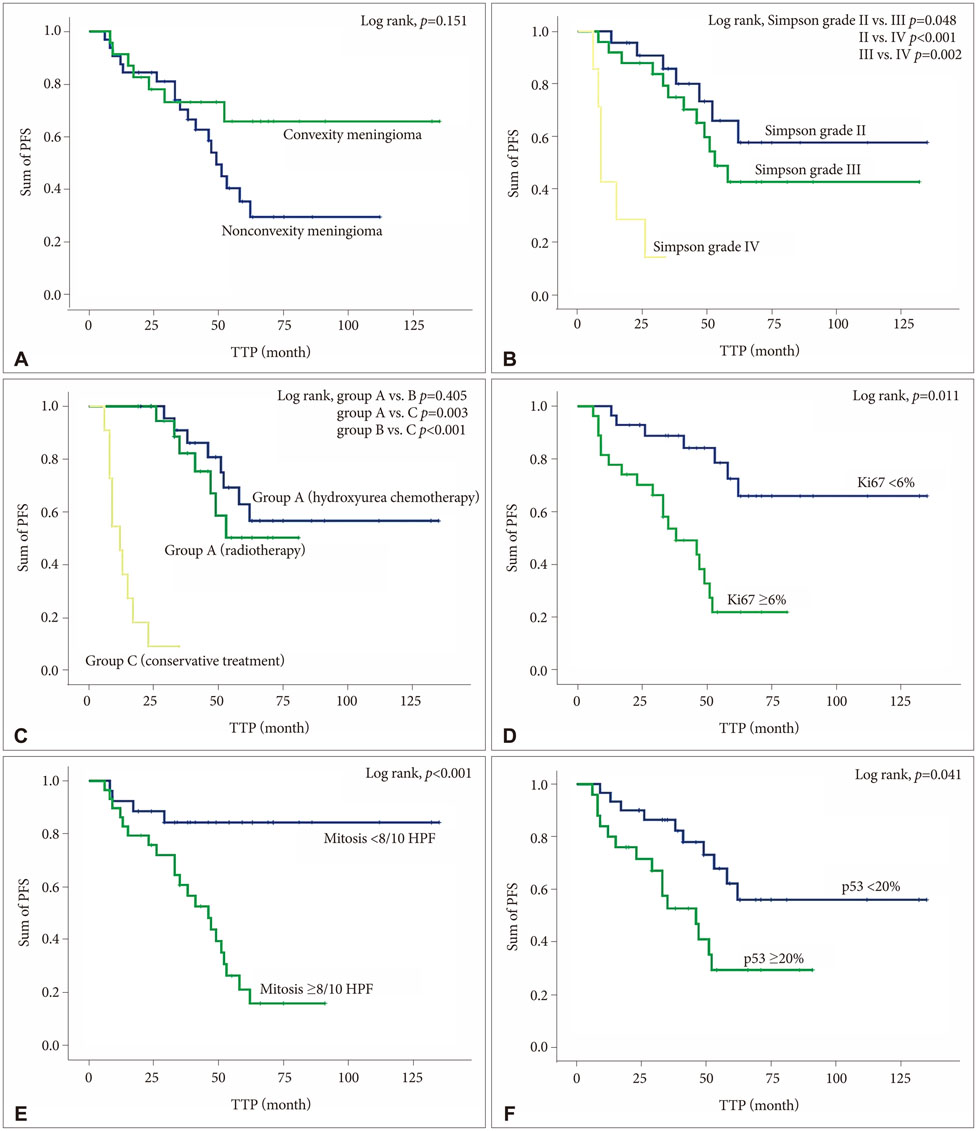
Among the 85 patients, 55 (65.5%) patients underwent incomplete resection; 24 (43.6%) were treated with adjuvant hydroxyurea (group A), and 20 (36.4%) with postoperative radiotherapy (group B), and 11 (20.0%) underwent conservative treatment after surgery (group C). Twenty-five (45.5%) patients experienced the progression of tumors during the follow-up period (mean 47.7 months, range 12.4-132.1 months); 8 of 24 (33.3%) patients in group A, 7 of 20 (35.0%) patients in group B, and 10 of 11 (90.9%) patients in group C. The mean progression-free survival (PFS) was 30.9 months (range 6.4-62.3 months); 46.2 months in group A, 40.4 months in group B, and 11.9 months in group C (p=0.041). Multivariate analysis showed that Simpson grade (p=0.040), adjuvant treatment after surgery (p<0.001), increased Ki67 (p=0.017), mitotic index (p=0.034), and overexpression of p53 (p=0.026) predicted longer PFS.
CONCLUSION
This investigation suggested that adjuvant treatment after incomplete resection of ATMNGs are associated with longer PFS than conservative treatment, and that there is no difference of PFS between hydroxyurea chemotherapy and radiotherapy after surgery. Therefore, hydroxyurea chemotherapy can be considered as another adjuvant tool for the ATMNGs if the postoperative adjuvant radiotherapy cannot be applicable.
MeSH Terms
Figure
Reference
-
1. Ostrom QT, Gittleman H, Xu J, et al. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2009-2013. Neuro Oncol. 2016; 18:suppl_5. v1–v75.
Article2. Dho YS, Jung KW, Ha J, et al. An updated nationwide epidemiology of primary brain tumors in Republic of Korea, 2013. Brain Tumor Res Treat. 2017; 5:16–23.
Article3. Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016; 131:803–820.
Article4. Rogers L, Gilbert M, Vogelbaum MA. Intracranial meningiomas of atypical (WHO grade II) histology. J Neurooncol. 2010; 99:393–405.
Article5. Brat DJ, Parisi JE, Kleinschmidt-DeMasters BK, et al. Surgical neuropathology update: a review of changes introduced by the WHO classification of tumours of the central nervous system, 4th edition. Arch Pathol Lab Med. 2008; 132:993–1007.
Article6. Perry A, Rushing EJ, Louise DN. Meningiomas. In : Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Ellison DW, Figarella-Branger D, editors. WHO Classification of Tumours of the Central Nervous System. 4th ed. Lyon: IARC Press;2016. p. 232–245. vol. 1.7. Kane AJ, Sughrue ME, Rutkowski MJ, et al. Anatomic location is a risk factor for atypical and malignant meningiomas. Cancer. 2011; 117:1272–1278.
Article8. Choy W, Kim W, Nagasawa D, et al. The molecular genetics and tumor pathogenesis of meningiomas and the future directions of meningioma treatments. Neurosurg Focus. 2011; 30:E6.
Article9. NCCN Clinical Practice Guidelines in Oncology (NCCN Guideline®). Central Nervous System Cancers. ver. 1.2016. NCCN.org;2016. Accessed May 17, 2017. at https://www.nccn.org/store/login/login.aspx?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf.10. Brem SS, Bierman PJ, Brem H, et al. Central nervous system cancers: clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2011; 9:352–400.
Article11. Schrell UM, Rittig MG, Anders M. Hydroxyurea for treatment of unresectable and recurrent meningiomas. II. Decrease in the size of meningiomas in patients treated with hydroxyurea. J Neurosurg. 1997; 86:840–844.
Article12. Mason WP, Gentili F, Macdonald DR, Hariharan S, Cruz CR, Abrey LE. Stabilization of disease progression by hydroxyurea in patients with recurrent or unresectable meningioma. J Neurosurg. 2002; 97:341–346.
Article13. Hahn BM, Schrell UM, Sauer R, Fahlbusch R, Ganslandt O, Grabenbauer GG. Prolonged oral hydroxyurea and concurrent 3d-conformal radiation in patients with progressive or recurrent meningioma: results of a pilot study. J Neurooncol. 2005; 74:157–165.
Article14. Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990; 8:1277–1280.
Article15. Moazzam AA, Wagle N, Zada G. Recent developments in chemotherapy for meningiomas: a review. Neurosurg Focus. 2013; 35:E18.
Article16. Lee EJ, Kim JH, Park ES, et al. A novel weighted scoring system for estimating the risk of rapid growth in untreated intracranial meningiomas. J Neurosurg. 2017; DOI: 10.3171/2016.9.JNS161669. [Epub].17. Chamberlain MC, Barnholtz-Sloan JS. Medical treatment of recurrent meningiomas. Expert Rev Neurother. 2011; 11:1425–1432.
Article18. Newton HB, Scott SR, Volpi C. Hydroxyurea chemotherapy for meningiomas: enlarged cohort with extended follow-up. Br J Neurosurg. 2004; 18:495–499.
Article19. Newton HB, Slivka MA, Stevens C. Hydroxyurea chemotherapy for unresectable or residual meningioma. J Neurooncol. 2000; 49:165–170.20. Loven D, Hardoff R, Sever ZB, et al. Non-resectable slow-growing meningiomas treated by hydroxyurea. J Neurooncol. 2004; 67:221–226.
Article21. Swinnen LJ, Rankin C, Rushing EJ, Laura HF, Damek DM, Barger GR. Phase II study of hydroxyurea for unresectable meningioma (Southwest Oncology Group S9811). J Clinical Oncol. 2009; 27:15.
Article22. Chamberlain MC. Medical and Neuro-oncology: NO-04. Hydroxyurea for recurrent surgery and radiation refractory meningioma. Neuro Oncol. 2010; 12:Suppl 4. iv36.23. Balik V, Sulla I, Park HH, Sarissky M. In vitro testing to a panel of potential chemotherapeutics and current concepts of chemotherapy in benign meningiomas. Surg Oncol. 2015; 24:292–299.
Article24. Champeaux C, Dunn L. World Health Organization grade II meningioma: a 10-year retrospective study for recurrence and prognostic factor assessment. World Neurosurg. 2016; 89:180–186.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Long-Term Follow-Up Result of Hydroxyurea Chemotherapy for Recurrent Meningiomas
- Evaluation of the Migration Behavior in Meningioma-derived Primary Cell Cultures by in Vitro Assay
- Correlation of histopathologic classification with proliferative activity and DNA ploidy in 120 intracranial meningiomas, with special reference to atypical meningioma
- Hydroxyurea-induced Multiple Skin Ulcers
- Correlation between Expression of p53 Protein and Prognostic Factors in Meningiomas