Yonsei Med J.
2008 Feb;49(1):53-57.
First Outbreak of Multidrug-Resistant Klebsiella pneumoniae Producing both SHV-12-Type Extended-Spectrum beta-Lactamase and DHA-1-Type AmpC beta-Lactamase at a Korean Hospital
- Affiliations
-
- 1Department of Laboratory Medicine, Korea University Medical College, Seoul, Korea. swonkeun@hallym.or.kr
- 2Department of Laboratory Medicine, Yonsei Wonju University College of Medicine, Wonju, Korea.
- 3Department of Laboratory Medicine, Hallym University College of Medicine, Seoul, Korea.
- 4Research Institute of Bacterial Resistance, Yonsei University College of Medicine, Seoul, Korea.
Abstract
- PURPOSE
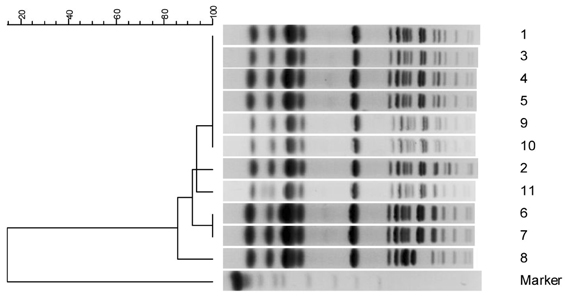
Coexistence of different classes of beta-lactamases in a single bacterial isolate may pose diagnostic and therapeutic challenges. We investigated a spread of Klebsiella pneumoniae isolates co-producing an AmpC beta-lactamase and an extended-spectrum beta-lactamase (ESBL) in a university hospital. MATERIALS AND METHODS: Over a three-month period, a total of 11 K. pneumoniae isolates, which exhibited resistance to cefotaxime, aztreonam, and cefoxitin, were isolated. These isolates showed positive to ESBLs by double disk tests. Minimal inhibitory concentrations (MICs) were determined by broth microdilution testing. All isolates were examined by isoelectric focusing, PCR and sequence analysis to identify bla(SHV) and bla(DHA), and molecular typing by pulsed-field gel electrophoresis (PFGE). RESULTS: All 11 isolates were highly resistant (MIC, > or = 128microngram/ml) to ceftazidime, aztreonam, and cefoxitin, while they were susceptible (MIC, < or = 2microngram/ml) to imipenem. The bla(SHV-12) and bla(DHA-1) genes were detected by PCR and sequence analysis. PFGE revealed a similar pattern in 10 of the 11 strains tested. CONCLUSION: This is the first outbreak report of K. pneumoniae in Korea which co-produced SHV-12 and DHA-1 beta-lactamase, and we suggest a clonal spread of multidrug-resistant K. pneumoniae at a hospital.
Keyword
MeSH Terms
-
Adult
Aged
Aged, 80 and over
*Disease Outbreaks
Disease Susceptibility
*Drug Resistance, Multiple, Bacterial
Female
Genotype
Hospitals
Humans
Klebsiella Infections/*epidemiology/*microbiology
Klebsiella pneumoniae/classification/*enzymology/genetics/isolation & purification
Korea
Male
Middle Aged
Phenotype
beta-Lactamases/*classification/genetics/*metabolism
Figure
Reference
-
1. Bradford PA. Extended-spectrum β-lactamases in the 21st century: characterization, epidemiology, and detection of this important resistance threat. Clin Microbiol Rev. 2001. 14:933–951.
Article2. Philippon A, Arlet G, Jacoby GA. Plasmid-determined AmpC-type β-lactamases. Antimicrob Agents Chemother. 2002. 46:1–11.
Article3. Ryoo NH, Kim EC, Hong SG, Park YJ, Lee K, Bae IK, et al. Dissemination of SHV-12 and CTX-M-type extended-spectrum β-lactamases among clinical isolates of Escherichia coli and Klebsiella pneumoniae and emergence of GES-3 in Korea. J Antimicrob Chemother. 2005. 56:698–702.
Article4. Lee K, Lee M, Shin JH, Lee MH, Kang SH, Park AJ, et al. Prevalence of plasmid-mediated AmpC β-lactamases in Escherichia coli and Klebsiella pneumoniae in Korea. Microb Drug Resist. 2006. 12:44–49.
Article5. Yan JJ, Ko WC, Wu HM, Tsai SH, Chuang CL, Wu JJ. Complexity of Klebsiella pneumoniae isolates resistant to both cephamycins and extended-spectrum cephalosporins at a teaching hospital in Taiwan. J Clin Microbiol. 2004. 42:5337–5340.
Article6. Moland ES, Hanson ND, Black JA, Hossain A, Song W, Thomson KS. Prevalence of newer β-lactamases in gram-negative clinical isolates collected in the United States from 2001 to 2002. J Clin Microbiol. 2006. 44:3318–3324.
Article7. Clinical and Laboratory Standards Institute. M100-S17. Performance standards for antimicrobial susceptibility testing; seventeenth informational supplement . 2007. Wayne, PA: Clinical and Laboratory Standards Institute.8. Brun-Buisson C, Legrand P, Philippon A, Montravers F, Ansquer M, Duval J. Transferable enzymatic resistance to third-generation cephalosporins during nosocomial outbreak of multiresistant Klebsiella pneumoniae. Lancet. 1987. 2:302–306.9. Clinical and Laboratory Standards Institute. M7-A7. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; Approved standard. 2006. 7th ed. Wayne, PA: Clinical and Laboratory Standards Institute.10. Black JA, Moland ES, Thomson KS. AmpC disk test for detection of plasmid-mediated AmpC β-lactamases in Enterobacteriaceae lacking chromosomal AmpC β-lactamases. J Clin Microbiol. 2005. 43:3110–3113.
Article11. Mathew A, Harris AM, Marshall MJ, Rose GW. The use of analytical isoelectric focusing for detection and identification of β-lactamases. J Gen Microbiol. 1975. 88:169–178.
Article12. Takahashi S, Nagano Y. Rapid procedure for isolation of plasmid DNA and application to epidemiological analysis. J Clin Microbiol. 1984. 20:608–613.
Article13. Pérez-Pérez FJ, Hanson ND. Detection of plasmid-mediated AmpC β-lactamase genes in clinical isolates by using multiplex PCR. J Clin Microbiol. 2002. 40:2153–2162.
Article14. Song W, Kim JS, Kim HS, Yong D, Jeong SH, Park MJ, et al. Increasing trend in the prevalence of plasmid-mediated AmpC β-lactamases in Enterobacteriaceae lacking chromosomal ampC gene at a Korean university hospital from 2002 to 2004. Diagn Microbiol Infect Dis. 2006. 55:219–224.
Article15. Livermore DM, Yuan M. Antibiotic resistance and production of extended-spectrum beta-lactamases amongst Klebsiella spp. from intensive care units in Europe. J Antimicrob Chemother. 1996. 38:409–424.
Article16. Kim J, Kwon Y, Pai H, Kim JW, Cho DT. Survey of Klebsiella pneumoniae strains producing extended-spectrum β-lactamases: prevalence of SHV-12 and SHV-2a in Korea. J Clin Microbiol. 1998. 36:1446–1449.
Article17. Pai H. The characteristics of extended-spectrum β-lactamases in Korean isolates of Enterobacteriaceae. Yonsei Med J. 1998. 39:514–519.18. Yum JH, Kim S, Lee H, Yong D, Lee K, Cho SN, et al. Emergence and wide dissemination of CTX-M-type ESBLs, and CMY-2 and DHA-1-type AmpC β-lactamases in Korean respiratory isolates of Klebsiella pneumoniae. J Korean Med Sci. 2005. 20:961–965.19. Gaillot O, Clément C, Simonet M, Philippon A. Novel transferable β-lactam resistance with cephalosporinase characteristics in Salmonella enteritidis. J Antimicrob Chemother. 1997. 39:85–87.
Article20. Verdet C, Benzerara Y, Gautier V, Adam O, Ould-Hocine Z, Arlet G. Emergence of DHA-1-producing Klebsiella spp. in the Parisian region: genetic organization of the ampC and ampR genes originating Morganella morganii. Antimicrob Agents Chemother. 2006. 50:607–617.
Article21. Moland ES, Black JA, Ourada J, Reisbig MD, Hanson ND, Thomson KS. Occurrence of newer β-lactamases in Klebsiella pneumoniae isolates from 24 U.S. hospitals. Antimicrob Agents Chemother. 2002. 46:3837–3842.
Article22. Song W, Lee KM, Kim HS, Kim JS, Kim J, Jeong SH, et al. Clonal spread of both oxyimino-cephalosporin- and cefoxitin-resistant Klebsiella pneumoniae isolates co-producing SHV-2a and DHA-1 β-lactamase at a burns intensive care unit. Int J Antimicrob Agents. 2006. 28:520–524.
Article23. Muratani T, Kobayashi T, Matsumoto T. Emergence and prevalence of β-lactamase-producing Klebsiella pneumoniae resistant to cephems in Japan. Int J Antimicrob Agents. 2006. 27:491–499.
Article24. De Gheldre Y, Struelens MJ, Glupczynski Y, De Mol P, Maes N, Nonhoff C, et al. National epidemiologic surveys of Enterobacter aerogenes in Belgian hospitals from 1996 to 1998. J Clin Microbiol. 2001. 39:889–896.
Article25. Thomson KS, Prevan AM, Sanders CC. Novel plasmid-mediated β-lactamases in enterobacteriaceae: emerging problems for new β-lactam antibiotics. Curr Clin Top Infect Dis. 1996. 16:151–163.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Characterization of Extended-spectrum Beta-lactamases in Klebsiella pneumoniae Isolated in Korea
- Frequency of Extended-spectrum beta-lactamase (ESBL) and AmpC beta-lactamase Genes in Escherichia coli and Klebsiella pneumoniae over a Three-year Period in a University Hospital in Korea
- In Vitro Susceptibility of piperacillin/tazobactam Against extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae
- Two Cases of Neonatal Osteomyelitis due to Extended Spectrum beta-lactamase Producing Klebsiella pneumoniae
- Prevalence of TEM- and SHV-type Beta-lactamase gene in Escherichia coli and Klebsiella pneumoniae in Korea