Cancer Res Treat.
2017 Oct;49(4):1114-1126. 10.4143/crt.2016.528.
Education Level Is a Strong Prognosticator in the Subgroup Aged More Than 50 Years Regardless of the Molecular Subtype of Breast Cancer: A Study Based on the Nationwide Korean Breast Cancer Registry Database
- Affiliations
-
- 1Department of Surgery, SMG-SNU Boramae Medical Center, Seoul, Korea. kiterius@snu.ac.kr
- 2Department of Surgery, Korea Cancer Center Hospital, Seoul, Korea.
- 3Department of Surgery, Dong-A University Medical Center, Busan, Korea.
- 4Department of Surgery, Samsung Medical Center, Seoul, Korea.
- 5Department of Surgery, Chonnam National University Medical School, Gwangju, Korea.
- 6Department of Surgery, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 7Saegyaero Hospital, Busan, Korea.
- 8Department of Biostatistics, SMG-SNU Boramae Medical Center, Seoul, Korea.
- 9Department of Pathology, SMG-SNU Boramae Medical Center, Seoul, Korea.
- KMID: 2394830
- DOI: http://doi.org/10.4143/crt.2016.528
Abstract
- PURPOSE
This study investigated the role of the education level (EL) as a prognostic factor for breast cancer and analyzed the relationship between the EL and various confounding factors.
MATERIALS AND METHODS
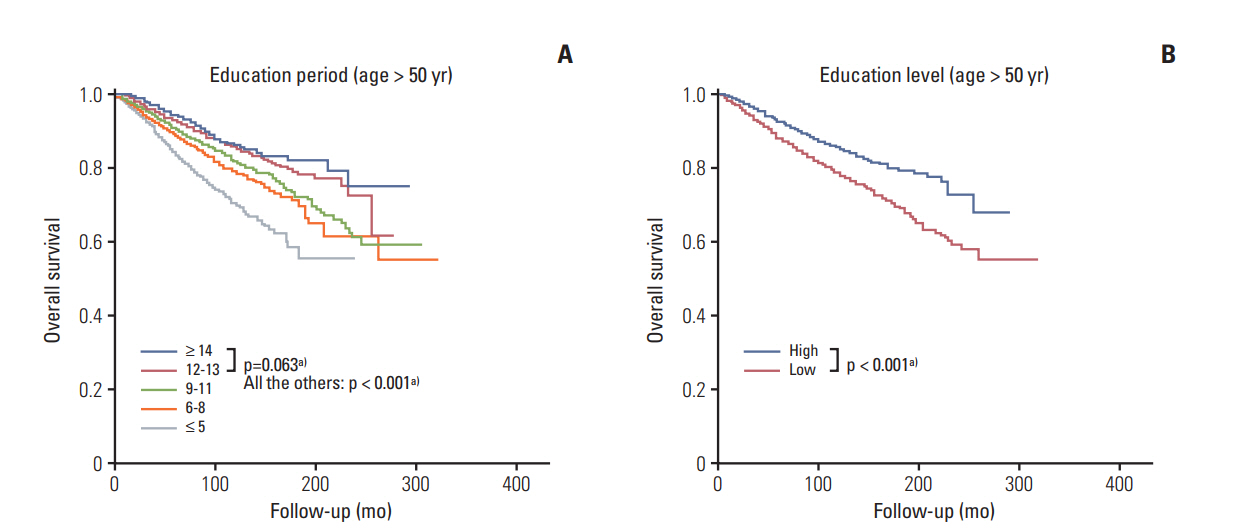
The data for 64,129 primary breast cancer patients from the Korean Breast Cancer Registry were analyzed. The EL was classified into two groups according to the education period; the high EL group (≥ 12 years) and low EL group (< 12 years). Survival analyses were performed with respect to the overall survival between the two groups.
RESULTS
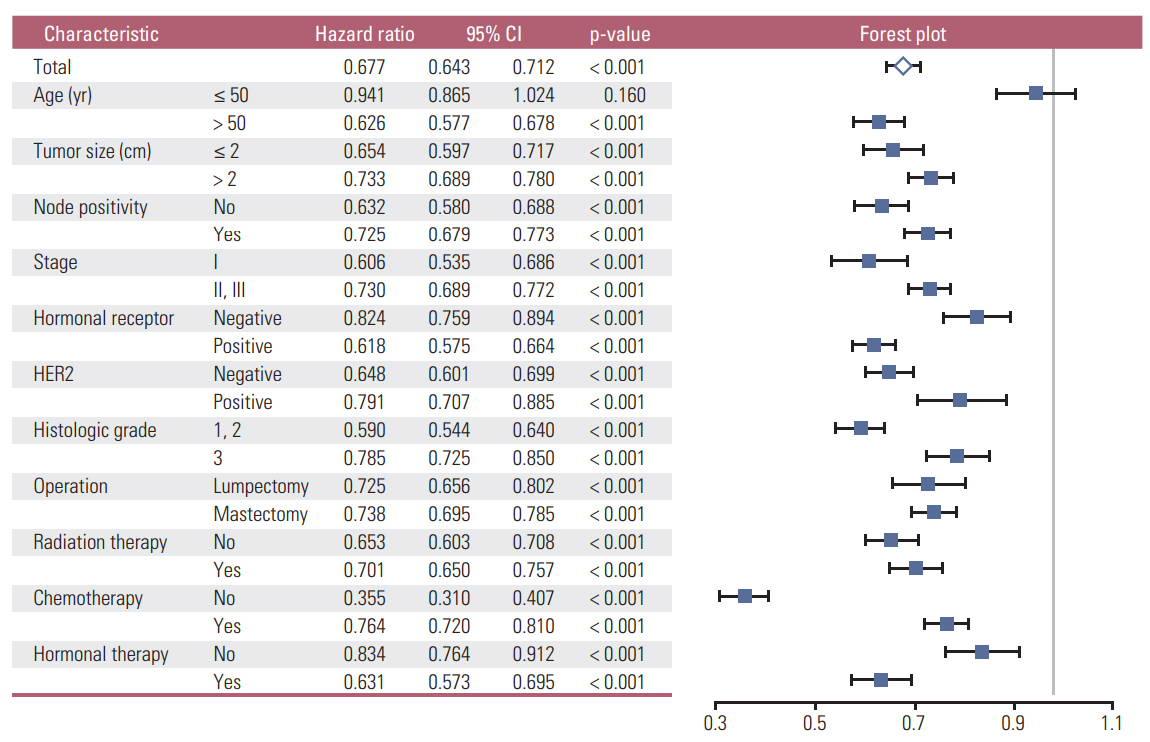
A high EL conferred a superior prognosis compared to a low EL in the subgroup aged > 50 years (hazard ratio, 0.626; 95% confidence interval [CI], 0.577 to 0.678) but not in the subgroup aged ≤ 50 years (hazard ratio, 0.941; 95% CI, 0.865 to 1.024). The EL was a significant independent factor in the subgroup aged > 50 years according to multivariate analyses. The high EL group showed more favorable clinicopathologic features and a higher proportion of patients in this group received lumpectomy, radiation therapy, and endocrine therapy. In the high EL group, a higher proportion of patients received chemotherapy in the subgroups with unfavorable clinicopathologic features. The EL was a significant prognosticator across all molecular subtypes of breast cancer.
CONCLUSION
The EL is a strong independent prognostic factor for breast cancer in the subgroup aged > 50 years regardless of the molecular subtype, but not in the subgroup aged ≤ 50 years. Favorable clinicopathologic features and active treatments can explain the main causality of the superior prognosis in the high EL group.
MeSH Terms
Figure
Cited by 2 articles
-
Prognostic influence of Korean public medical insurance system on breast cancer patients
Ki-Tae Hwang, Young Wook Ju, Young A Kim, Jongjin Kim, Sohee Oh, Jiwoong Jung, Young Jun Chai, In Sil Choi, So Won Oh
Ann Surg Treat Res. 2019;96(2):58-69. doi: 10.4174/astr.2019.96.2.58.Risk factors for intensive care unit readmission after lung transplantation: a retrospective cohort study
Hye-Bin Kim, Sungwon Na, Hyo Chae Paik, Hyeji Joo, Jeongmin Kim
Acute Crit Care. 2021;36(2):99-108. doi: 10.4266/acc.2020.01144.
Reference
-
References
1. Hussain SK, Lenner P, Sundquist J, Hemminki K. Influence of education level on cancer survival in Sweden. Ann Oncol. 2008; 19:156–62.
Article2. Hussain SK, Altieri A, Sundquist J, Hemminki K. Influence of education level on breast cancer risk and survival in Sweden between 1990 and 2004. Int J Cancer. 2008; 122:165–9.
Article3. Albano JD, Ward E, Jemal A, Anderson R, Cokkinides VE, Murray T, et al. Cancer mortality in the United States by education level and race. J Natl Cancer Inst. 2007; 99:1384–94.
Article4. Gajalakshmi CK, Shanta V, Swaminathan R, Sankaranarayanan R, Black RJ. A population-based survival study on female breast cancer in Madras, India. Br J Cancer. 1997; 75:771–5.
Article5. Lund E, Jacobsen BK. Education and breast cancer mortality: experience from a large Norwegian cohort study. Cancer Causes Control. 1991; 2:235–8.
Article6. Nishi N, Sugiyama H, Hsu WL, Soda M, Kasagi F, Mabuchi K, et al. Differences in mortality and incidence for major sites of cancer by education level in a Japanese population. Ann Epidemiol. 2008; 18:584–91.
Article7. Robsahm TE, Tretli S. Weak associations between sociodemographic factors and breast cancer: possible effects of early detection. Eur J Cancer Prev. 2005; 14:7–12.
Article8. Heck KE, Wagener DK, Schatzkin A, Devesa SS, Breen N. Socioeconomic status and breast cancer mortality, 1989 through 1993: an analysis of education data from death certificates. Am J Public Health. 1997; 87:1218–22.
Article9. Steenland K, Henley J, Thun M. All-cause and cause-specific death rates by educational status for two million people in two American Cancer Society cohorts, 1959-1996. Am J Epidemiol. 2002; 156:11–21.
Article10. Kim C, Eby E, Piette JD. Is education associated with mortality for breast cancer and cardiovascular disease among black and white women? Gend Med. 2005; 2:13–8.
Article11. Dano H, Hansen KD, Jensen P, Petersen JH, Jacobsen R, Ewertz M, et al. Fertility pattern does not explain social gradient in breast cancer in denmark. Int J Cancer. 2004; 111:451–6.12. Min SY, Kim Z, Hur MH, Yoon CS, Park EH, Jung KW, et al. The basic facts of Korean breast cancer in 2013: results of a nationwide survey and breast cancer registry database. J Breast Cancer. 2016; 19:1–7.
Article13. Rutqvist LE, Bern A; Stockholm Breast Cancer Study Group. Socioeconomic gradients in clinical stage at presentation and survival among breast cancer patients in the Stockholm area 1977-1997. Int J Cancer. 2006; 119:1433–9.
Article14. Cella DF, Orav EJ, Kornblith AB, Holland JC, Silberfarb PM, Lee KW, et al. Socioeconomic status and cancer survival. J Clin Oncol. 1991; 9:1500–9.
Article15. Herndon JE 2nd, Kornblith AB, Holland JC, Paskett ED. Effect of socioeconomic status as measured by education level on survival in breast cancer clinical trials. Psychooncology. 2013; 22:315–23.16. Mackenbach JP, Kunst AE, Groenhof F, Borgan JK, Costa G, Faggiano F, et al. Socioeconomic inequalities in mortality among women and among men: an international study. Am J Public Health. 1999; 89:1800–6.
Article17. Faggiano F, Lemma P, Costa G, Gnavi R, Pagnanelli F. Cancer mortality by educational level in Italy. Cancer Causes Control. 1995; 6:311–20.
Article18. Dalton SO, During M, Ross L, Carlsen K, Mortensen PB, Lynch J, et al. The relation between socioeconomic and demographic factors and tumour stage in women diagnosed with breast cancer in Denmark, 1983-1999. Br J Cancer. 2006; 95:653–9.
Article19. Gordon NH. Association of education and income with estrogen receptor status in primary breast cancer. Am J Epidemiol. 1995; 142:796–803.
Article20. Griggs JJ, Culakova E, Sorbero ME, van Ryn M, Poniewierski MS, Wolff DA, et al. Effect of patient socioeconomic status and body mass index on the quality of breast cancer adjuvant chemotherapy. J Clin Oncol. 2007; 25:277–84.
Article21. Bouchardy C, Verkooijen HM, Fioretta G. Social class is an important and independent prognostic factor of breast cancer mortality. Int J Cancer. 2006; 119:1145–51.
Article22. Damiani G, Basso D, Acampora A, Bianchi CB, Silvestrini G, Frisicale EM, et al. The impact of level of education on adherence to breast and cervical cancer screening: Evidence from a systematic review and meta-analysis. Prev Med. 2015; 81:281–9.
Article23. Yu XQ. Socioeconomic disparities in breast cancer survival: relation to stage at diagnosis, treatment and race. BMC Cancer. 2009; 9:364.
Article24. Louwman WJ, van de Poll-Franse LV, Fracheboud J, Roukema JA, Coebergh JW. Impact of a programme of mass mammography screening for breast cancer on socio-economic variation in survival: a population-based study. Breast Cancer Res Treat. 2007; 105:369–75.
Article25. Baquet CR, Commiskey P. Socioeconomic factors and breast carcinoma in multicultural women. Cancer. 2000; 88:1256–64.
Article26. Patterson RE, Cadmus LA, Emond JA, Pierce JP. Physical activity, diet, adiposity and female breast cancer prognosis: a review of the epidemiologic literature. Maturitas. 2010; 66:5–15.
Article27. Hellmann SS, Thygesen LC, Tolstrup JS, Gronbaek M. Modifiable risk factors and survival in women diagnosed with primary breast cancer: results from a prospective cohort study. Eur J Cancer Prev. 2010; 19:366–73.
Article28. Reynolds P, Goldberg D, Hurley S, Nelson DO, Largent J, Henderson KD, et al. Passive smoking and risk of breast cancer in the California teachers study. Cancer Epidemiol Biomarkers Prev. 2009; 18:3389–98.
Article29. McDonald PA, Williams R, Dawkins F, Adams-Campbell LL. Breast cancer survival in African American women: is alcohol consumption a prognostic indicator? Cancer Causes Control. 2002; 13:543–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prognostic Influence of BCL2 on Molecular Subtypes of Breast Cancer
- The different prognostic impact of age according to individual molecular subtypes in breast cancer
- The Basic Facts of Korean Breast Cancer in 2013: Results of a Nationwide Survey and Breast Cancer Registry Database
- Surgery of Breast Cancer during the Last 5 Years: More Sophisticated and Specialized?
- The Highligts of 28th Annual Meeting of San Antonio Breast Cancer Symposium