J Korean Med Assoc.
2008 Mar;51(3):262-272.
Surgical Treatment of Epilepsy
- Affiliations
-
- 1Department of Neurosurgery, Seoul National University College of Medicine, Korea. chungc@snu.ac.kr
Abstract
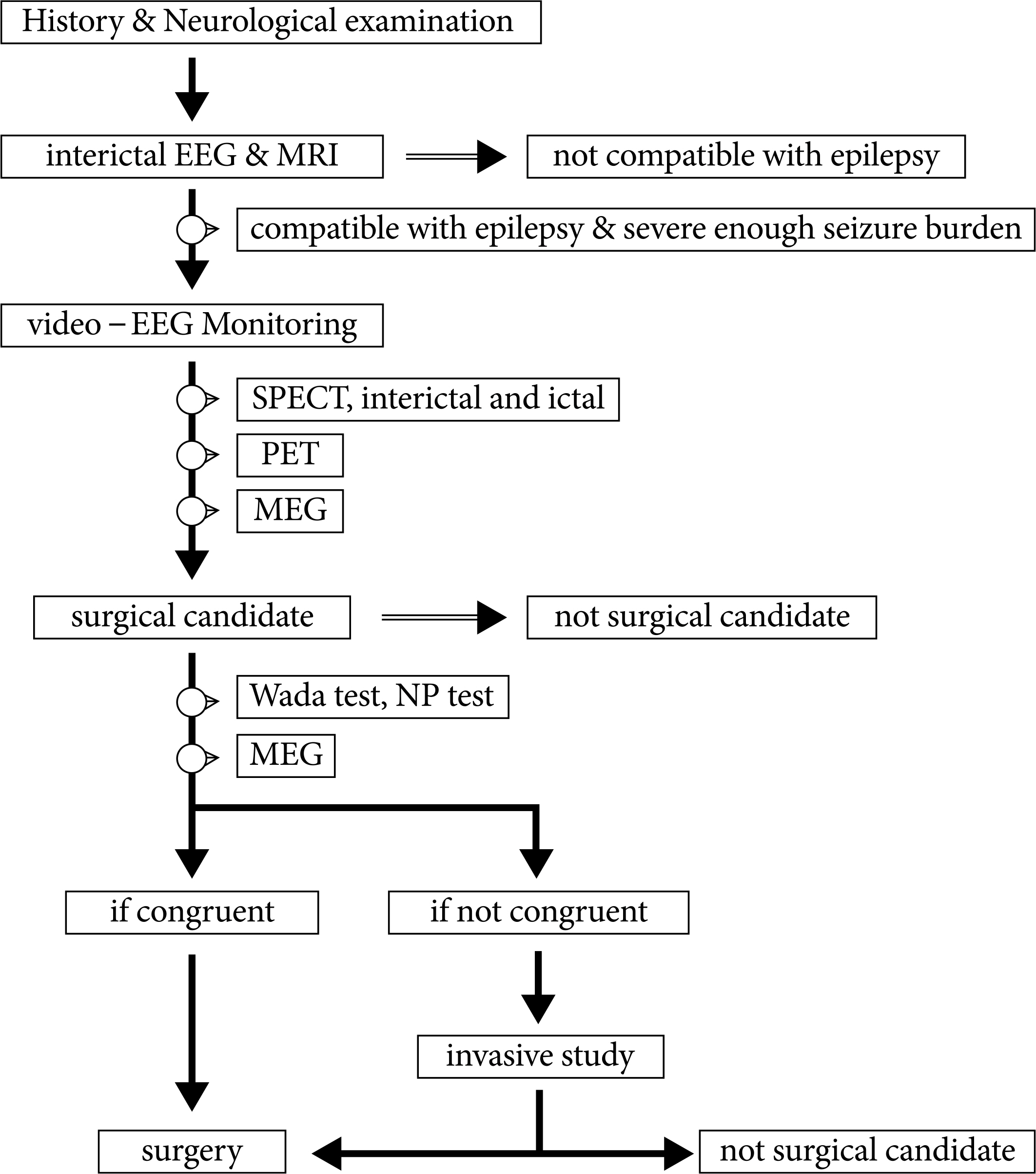
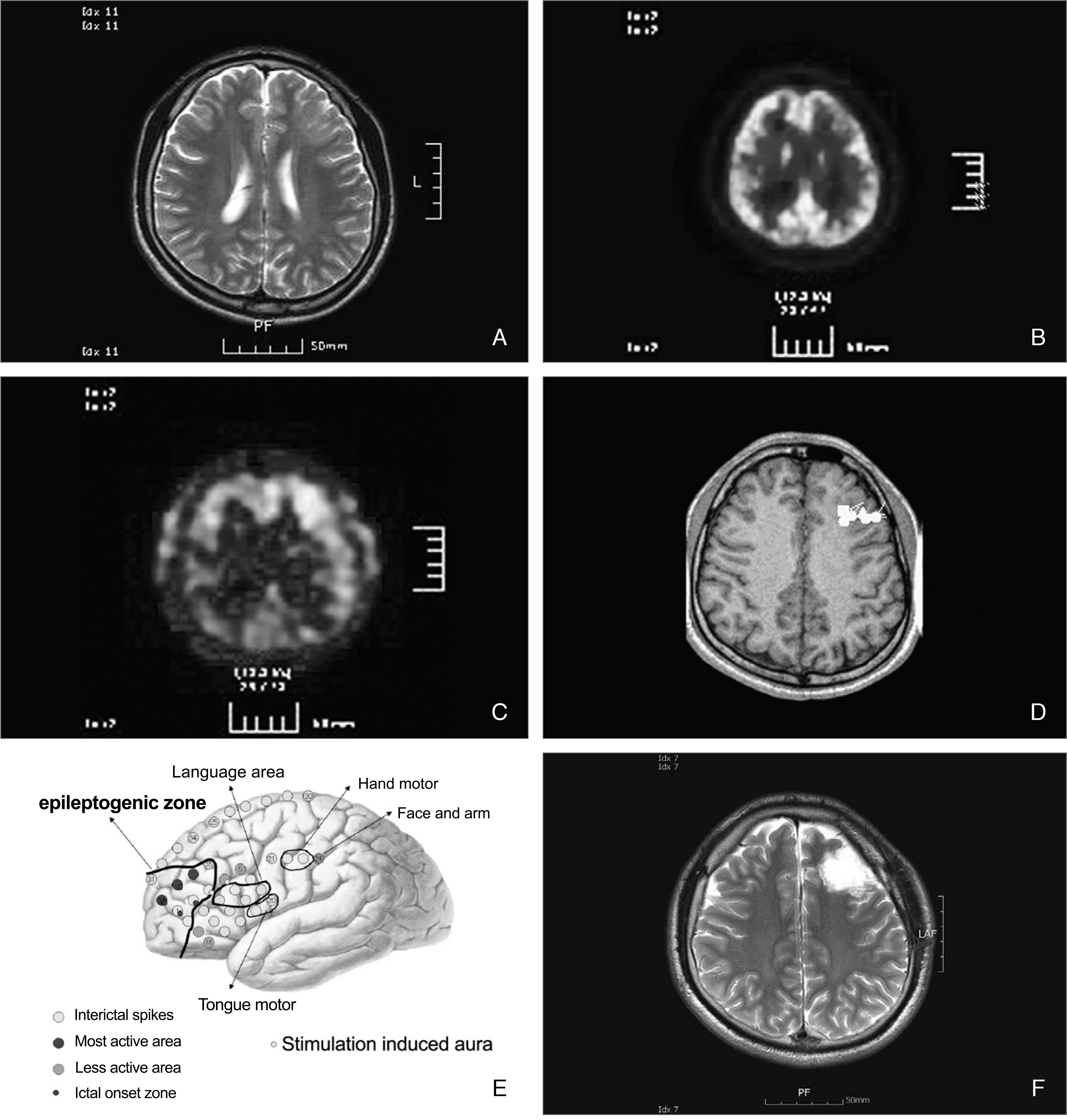
- More than 30% of epilepsy patients are not controlled by anti-epileptic medications. For patients having intractable epilepsy, epilepsy surgery is an effective treatment, which provides not only control of seizures but also improvement of quality of life. Epilepsy surgery can provide complete seizure control in over 60% of patients having medically intractable epilepsy. In order to identify surgical candidates, various diagnostic modalities are being used. The value of video-EEG monitoring and MR imaging study cannot be over-emphasized. For certain circumstances, other diagnostic modalities, such as PET, SPECT, and MEG, provide complementary data. If the findings from these non-invasive studies collectively indicate that the patient can benefit from surgery, surgical resection can be performed. However, if the findings do not,, invasive studies should follow. New surgical modalities for the treatment for epilepsy have been developed, including surgical resection of epileptogenic zone or lesion, disconnection of epileptogenic zone from the surrounding normal brain, and neuromodulation, such as vagal nerve stimulation, deep brain stimulation, etc. Also, newly emerging diagnostic modalities, such as high tesla MR imaging, magnetoencephalography or brain mapping technology, can help select surgical candidates more easily in the near future.
Keyword
MeSH Terms
Figure
Reference
-
References
1. Lee BI. Epilepsy: Epidemiology and Classification. J Korean Med Assoc. 2003; 46:269–278.
Article2. Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000; 342:314–319.
Article3. Wiebe S, Blume WT, Girvin JP, Eliasziw M. Effectiveness and efficiency of Surgery for Temporal Lobe Epilepsy Study Group. A randomized, controlled trial of surgery for temporal?lobe epilepsy. N Engl J Med. 2001; 345:311–318.
Article4. Engel J Jr. Surgery for seizures. N Engl J Med. 1996; 334:647–652.
Article5. Yun CH, Lee SK, Lee SY, Kim KK, Jeong SW, Chung CK. Prognostic factors in neocortical epilepsy surgery: multivariate analysis. Epilepsi. 2006; 47:574–579.
Article6. Willmann O, Wennberg R, May T, Woermann FG, Pohlmann-Eden B. The role of 1H magnetic resonance spectroscopy in pre?operative evaluation for epilepsy surgery. A meta? analysis. Epilepsy Res. 2006; 71:149–158.7. Akhtari M, Salamon N, Duncan R, Fried I, Mathern GW. Electrical conductivities of the freshly excised cerebral cortex in epilepsy surgery patients; correlation with pathology, seizure duration, and diffusion tensor imaging. Brain Topogr. 2006; 18:281–290.
Article8. Chandra PS, Salamon N, Huang J, Wu JY, Koh S, Vinters HV, Mathern GW. FDG?PET/MRI coregistration and diffusion? tensor imaging distinguish epileptogenic tubers and cortex in patients with tuberous sclerosis complex: a preliminary report. Epilepsia. 2006; 47:1543–1549.9. Powell HW, Parker GJ, Alexander DC, Symms MR, Boulby PA, Wheeler?Kingshott CA, Barker GJ, Koepp MJ, Duncan JS. Abnormalities of language networks in temporal lobe epilepsy. Neuroimage. 2007; 36:209–221.
Article10. Zijlmans M, Huiskamp G, Hersevoort M, Seppenwoolde JH, van Huffelen AC, Leijten FS. EEG?fMRI in the preoperative work?up for epilepsy surgery. Brain. 2007; 130:2343–2353.
Article11. Krakow K, Woermann FG, Symms MR, Allen PJ, Lemieux L, Barker GJ, Duncan JS, Fish DR. EEG?triggered functional MRI of interictal epileptiform activity in patients with partial seizures. Brain. 1999; 122:1679–1688.
Article12. Kim YK, Lee DS, Lee SK, Kim SK, Chung CK, Chang KH, Choi KY, Chung JK, Lee MC. Differential features of metabolic abnormalities between medial and lateral temporal lobe epilepsy: quantitative analysis of (18)F?FDG PET using SPM. J Nucl Med. 2003; 44:1006–1012.13. Kim YK, Lee DS, Lee SK, Chung CK, Chung JK, Lee MC. (18)F?FDG PET in localization of frontal lobe epilepsy: comparison of visual and SPM analysis. J Nucl Med. 2002; 43:1167–1174.14. Lee SK, Lee DS, Yeo JS, Lee JS, Kim YK, Jang MJ, Kim KK, Kim SK, Oh JB, Chung CK. FDG?PET images quantified by probabilistic atlas of brain and surgical prognosis of temporal lobe epilepsy. Epilepsia. 2002; 43:1032–1038.
Article15. Hammers A, Koepp MJ, Hurlemann R, Thom M, Richardson MP, Brooks DJ, Duncan JS. Abnormalities of grey and white matter [11C]flumazenil binding in temporal lobe epilepsy with normal MRI. Brain. 2002; 125:2257–2271.
Article16. Lee DS, Lee SK, Kim SK, Kang KW, Kang E, Lee KH, Hyun IY, Chung J, Lee MC. Late postictal residual perfusion abnormality in epileptogenic zone found on 6?hour postictal SPECT. Neurology. 2000; 55:835–841.
Article17. Lee SK, Lee SH, Kim SK, Lee DS, Kim H. The clinical usefulness of ictal SPECT in temporal lobe epilepsy: the lateralization of seizure focus and correlation with EEG. Epilepsia. 2000; 41:955–962.
Article18. Barkley GL, Baumgartner C. MEG and EEG in epilepsy. J Clin Neurophysiol. 2003; 20:163–178.
Article19. Knowlton RC, Shih J. Magnetoencephalography in epilepsy. Epilepsia. 2004; 45(S4):61–71.
Article20. Kim JS, Chung CK. Robust source analysis of oscillatory motor cortex activity with inherently variable phase delay. Neuroimage. 2007; 37:518–529.
Article21. Lee SK, Kim KK, Nam H, Oh JB, Yun CH, Chung CK. Adding or repositioning intracranial electrodes during presurgical assessment of neocortical epilepsy: electrographic seizure pattern and surgical outcome. J Neurosurg. 2004; 100:463–471.
Article22. Pre?surgical evaluation for epilepsy surgery?European standards. European Federation of Neurological Societies Task Force. Eur J Neurol. 2000; 7:119–122.23. French JA, Williamson PD, Thadani VM, Darcey TM, Mattson RH, Spencer SS, Spencer DD. Characteristics of medial temporal lobe epilepsy: I. Results of history and physical examination. Ann Neurol. 1993; 34:774–780.
Article24. Williamson PD, French JA, Thadani VM, Kim JH, Novelly RA, Spencer SS, Spencer DD, Mattson RH. Characteristics of medial temporal lobe epilepsy: II. Interictal and pubmed ictal scalp electroencephalography, neuropsychological testing, neuroimaging, surgical results, and pathology. Ann Neurol. 1993; 34:781–787.25. McIntosh AM, Kalnins RM, Mitchell LA, Fabinyi GC, Briell-mann RS, Berkovic SF. Temporal lobectomy: long?term seizure outcome, late recurrence and risks for seizure recurrence. Brain. 2004; 127:2018–2030.
Article26. Jeong SW, Lee SK, Hong KS, Kim KK, Chung CK, Kim H. Prognostic factors for the surgery for mesial temporal lobe epilepsy: longitudinal analysis. Epilepsia. 2005; 46:1273–1279.
Article27. Jeong SW, Lee SK, Kim KK, Kim H, Kim JY, Chung CK. Prognostic factors in anterior temporal lobe resections for mesial temporal lobe epilepsy: multivariate analysis. Epilepsia. 1999; 40:1735–1739.
Article28. Paolicchi JM. Can early epilepsy surgery in infants improve their developmental outcome? Nature Clinical Practice Neurology. 2007; 3:662–663.
Article29. Tinuper P, Andermann F, Villemure JG, Rasmussen TB, Ques-ney LF. Functional hemispherectomy for treatment of epilepsy associated with hemiplegia: rationale, indications, results, and comparison with callosotomy. Ann Neurol. 1988; 24:27–34.
Article30. Villemure JG, Mascott CR. Peri?insular hemispherotomy: surgical principles and anatomy. Neurosurgery. 1995; 37:975–981.31. Schramm J, Kral T, Clusmann H. Transsylvian keyhole functional hemispherectomy. Neurosurgery. 2001; 49:891–900. discussion 900–901.
Article32. Morrell F, Whisler WW, Bleck TP. Multiple subpial transection: a new approach to the surgical treatment of focal epilepsy. J Neurosurg. 1989; 70:231–239.
Article33. A randomized controlled trial of chronic vagus nerve stimulation for treatment of medically intractable seizures. The Vagus Nerve Stimulation Study Group. Neurology. 1995; 45:224–230.34. Landy HJ, Ramsay RE, Slater J, Casiano RR, Morgan R. Vagus nerve stimulation for complex partial seizures: surgical technique, safety, and efficacy. J Neurosurg. 1993; 78:26–31.
Article35. Salinsky MC, Uthman BM, Ristanovic RK, Wernicke JF, Tarver WB. Vagus nerve stimulation for the treatment of medically intractable seizures. Results of a 1?year open?extension trial. Vagus Nerve Stimulation Study Group. Arch Neurol. 1996; 53:1176–1180.36. Handforth A, DeGiorgio CM, Schachter SC, Uthman BM, Naritoku DK, Tecoma ES, Henry TR, Collins SD, Vaughn BV, Gilmartin RC, Labar DR, Morris GL 3rd, Salinsky MC, Osorio I, Ristanovic RK, Labiner DM, Jones JC, Murphy JV, Ney GC, Wheless JW. Vagus nerve stimulation therapy for partial? onset seizures: a randomized active?control trial. Neurology. 1998; 51:48–55.37. Labar D, Murphy J, Tecoma E. Vagus nerve stimulation for medication?resistant generalized epilepsy. E04 VNS Study Group. Neurology. 1999; 52:1510–1512.38. Murphy JV. Left vagal nerve stimulation in children with medically refractory epilepsy. The Pediatric VNS Study Group. J Pediatr. 1999; 134:563–566.39. Tellez?Zenteno JF, McLachlan RS, Parrent A, Kubu CS, Wiebe S. Hippocampal electrical stimulation in mesial temporal lobe epilepsy. Neurology. 2006; 66:1490–1494.
Article40. Andrade DM, Zumsteg D, Hamani C, Hodaie M, Sarkissian S, Lozano AM, Wennberg RA. Long?term follow?up of patients with thalamic deep brain stimulation for epilepsy. Neurology. 2006; 66:1571–1573.
Article41. Boon P, Vonck K, De Herdt V, Van Dycke A, Goethals M, Goossens L, Van Zandijcke M, De Smedt T, Dewaele I, Achten R, Wadman W, Dewaele F, Caemaert J, Van Roost D. Deep brain stimulation in patients with refractory temporal lobe epilepsy. Epilepsia. 2007; 48:1551–1560.
Article42. Téllez?Zenteno JF, Dhar R, Wiebe S. Long?term seizure outcomes following epilepsy surgery: a systematic review and meta?analysis. Brain. 2005; 128:1188–1198.43. Téllez?Zenteno JF, Dhar R, Hernandez?Ronquillo L, Wiebe S. Long?term outcomes in epilepsy surgery: antiepileptic drugs, mortality, cognitive and psychosocial aspects. Brain. 2007; 130:334–345.
Article44. Spencer SS, Berg AT, Vickrey BG, Sperling MR, Bazil CW, Haut S, Langfitt JT, Walczak TS, Devinsky O. Multicenter Study of Epilepsy Surgery. Health?related quality of life over time since resective epilepsy surgery. Ann Neurol. 2007; 62:327–334.
Article45. Hitiris N, Mohanraj R, Norrie J, Brodie MJ. Mortality in epilepsy. Epilepsy Behav. 2007; 10:363–376.
Article