J Pathol Transl Med.
2017 Sep;51(5):482-487. 10.4132/jptm.2017.07.11.
Acid-Fastness of Histoplasma in Surgical Pathology Practice
- Affiliations
-
- 1Department of Pathology, All India Institute of Medical Sciences, New Delhi, India. deepalijain76@gmail.com
- 2Department of Microbiology, All India Institute of Medical Sciences, New Delhi, India.
- KMID: 2392567
- DOI: http://doi.org/10.4132/jptm.2017.07.11
Abstract
- BACKGROUND
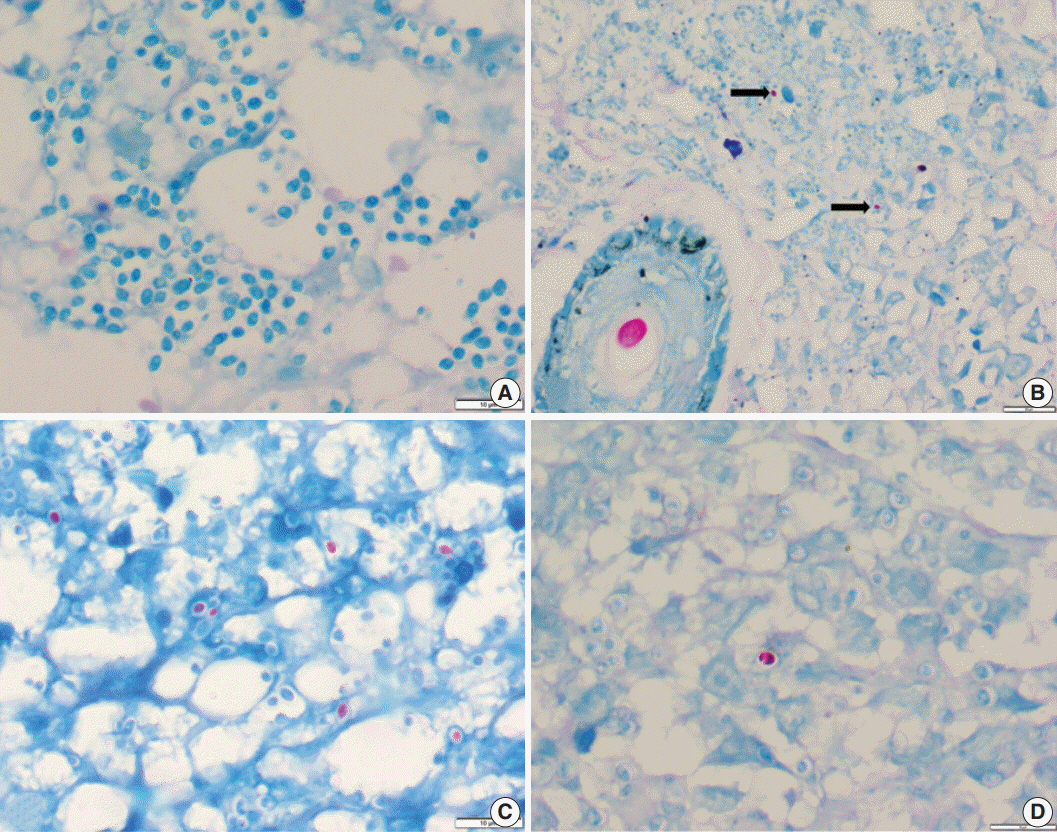
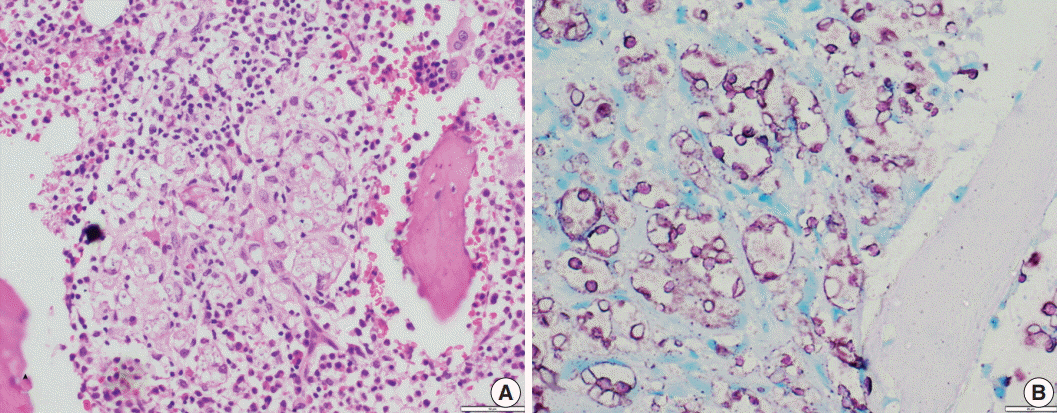
Histoplasmosis (HP) is diagnosed by visualizing intracellular microorganisms in biopsy and/or culture. Periodic-acid Schiff (PAS) and Gomori methenamine silver (GMS) staining methods are routinely used for identification. The acid-fast property of Histoplasma was identified decades ago, but acid-fast staining has not been practiced in current surgical pathology. Awareness of the acid-fast property of Histoplasma, which is due to mycolic acid in the cell wall, is important in distinguishing Histoplasma from other infective microorganisms. Here, we examined acid-fastness in previously diagnosed cases of Histoplasma using the Ziehl-Neelsen (ZN) stain and correlated those findings with other known fungal stains.
METHODS
All cases diagnosed as HP were retrieved and reviewed along with ZN staining and other fungal stains. We also stained cases diagnosed with Cryptococcus and Leishmania as controls for comparison.
RESULTS
A total of 54 patients ranging in age from 11 to 69 years were examined. The most common sites of infection were the skin, adrenal tissue, and respiratory tract. Of the total 43 tissue samples, 20 (46.5%) stained positive with the ZN stain. In viable cases, a significant proportion of microorganisms were positive while necrotic cases showed only rare ZN-positive yeasts. In comparison to PAS and GMS stains, there was a low burden of ZN-positive yeasts. Cryptococcus showed characteristic ZN staining and all cases of Leishmania were negative.
CONCLUSIONS
Although the morphology of fungal organisms is the foundation of identification, surgical pathologists should be aware of the acid-fast property of fungi, particularly when there is the potential for confusion with other infective organisms.
Keyword
MeSH Terms
Figure
Reference
-
1. Antinori S. Histoplasma capsulatum: more widespread than previously thought. Am J Trop Med Hyg. 2014; 90:982–3.2. Guimarães AJ, Nosanchuk JD, Zancopé-Oliveira RM. Diagnosis of histoplasmosis. Braz J Microbiol. 2006; 37:1–13.
Article3. Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011; 24:247–80.
Article4. Youngberg GA, Wallen ED, Giorgadze TA. Narrow-spectrum histochemical staining of fungi. Arch Pathol Lab Med. 2003; 127:1529–30.
Article5. Goswami RP, Pramanik N, Banerjee D, Raza MM, Guha SK, Maiti PK. Histoplasmosis in eastern India: the tip of the iceberg? Trans R Soc Trop Med Hyg. 1999; 93:540–2.
Article6. Gopalakrishnan R, Nambi PS, Ramasubramanian V, Abdul Ghafur K, Parameswaran A. Histoplasmosis in India: truly uncommon or uncommonly recognised? J Assoc Physicians India. 2012; 60:25–8.7. Kauffman CA. Histoplasmosis: a clinical and laboratory update. Clin Microbiol Rev. 2007; 20:115–32.
Article8. Azar MM, Hage CA. Laboratory diagnostics for histoplasmosis. J Clin Microbiol. 2017; 55:1612–20.
Article9. Joseph Wheat L. Current diagnosis of histoplasmosis. Trends Microbiol. 2003; 11:488–94.
Article10. Parsons RJ, Zarafonetis CJ. Histoplasmosis in man: report of seven cases and review of 71 cases. Arch Intern Med. 1945; 75:1–23.11. Rawson AJ. Acid-fast property of Histoplasma capsulatum. Am J Clin Pathol. 1948; 18:97.
Article12. Wages DS, Wear DJ. Acid-fastness of fungi in blastomycosis and histoplasmosis. Arch Pathol Lab Med. 1982; 106:440–1.13. Jain D. Acid fast property of histoplasma: a concept revitalized. Int J Surg Pathol. 2016; 24:724–5.14. Ranjan R, Jain D, Singh L, Iyer VK, Sharma MC, Mathur SR. Differentiation of histoplasma and cryptococcus in cytology smears: a diagnostic dilemma in severely necrotic cases. Cytopathology. 2015; 26:244–9.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Study on Histoplasmosis (Epizootic Lymphangitis) in Cart-Horses in Ethiopia
- Chronic Cavitary Pulmonary Histoplasmosis in a Non-HIV and Immunocompromised Patient without Overseas Travel History
- General Consideration on Uveitis
- A Case of Pulmonary Nocardiosis
- Disseminated Histoplasmosis and Tuberculosis in a Patient with HIV Infection