Allergy Asthma Respir Dis.
2017 Sep;5(5):248-255. 10.4168/aard.2017.5.5.248.
The roles of mast cells in allergic inflammation and mast cell-related disorders
- Affiliations
-
- 1Department of Internal Medicine, Kosin University College of Medicine, Busan, Korea. naum67@naver.com
- KMID: 2392399
- DOI: http://doi.org/10.4168/aard.2017.5.5.248
Abstract
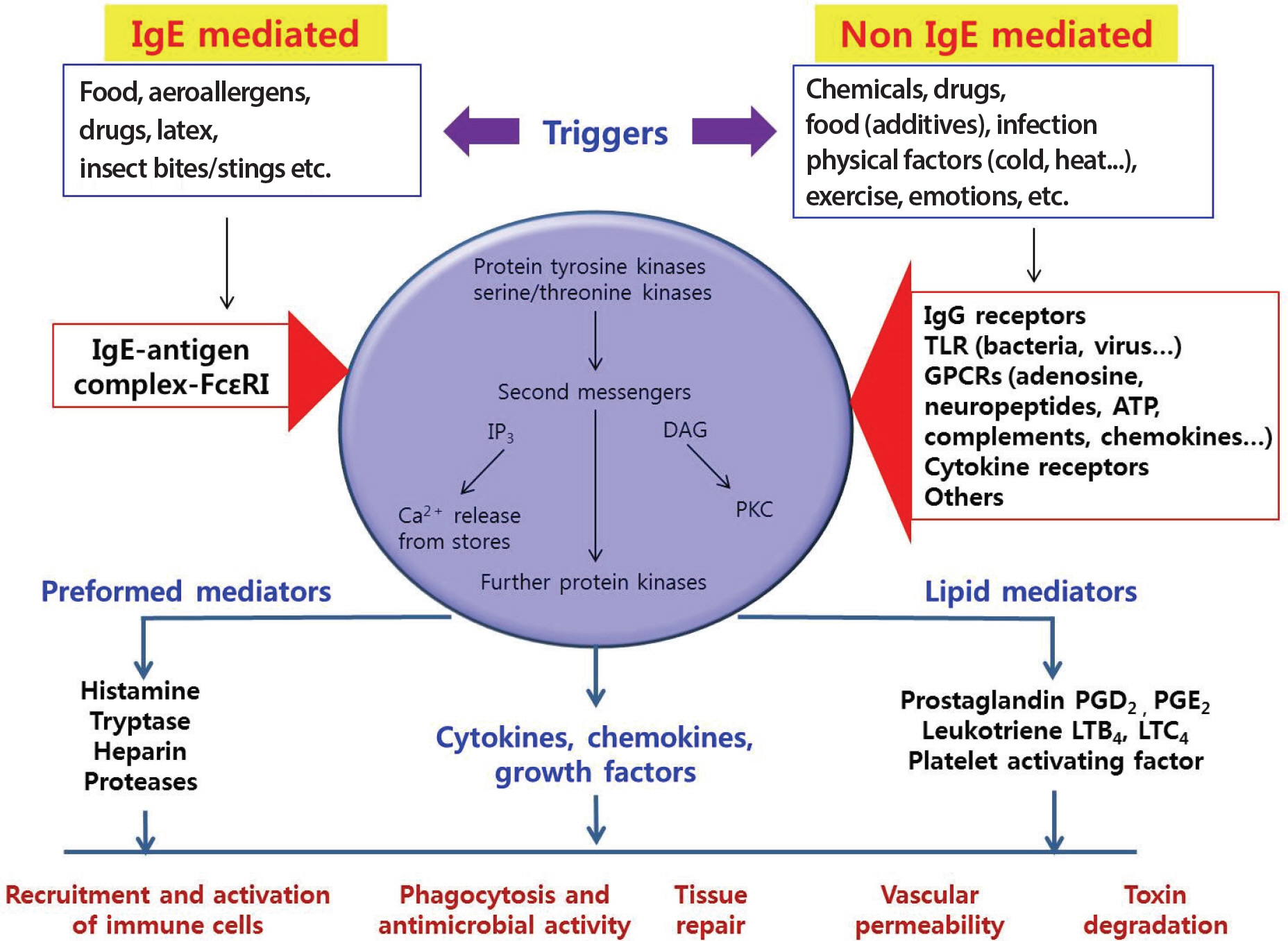
- Mast cells, which are major effector cells in allergic reactions, are found in the perivascular spaces of most tissues and contain pro-inflammatory and vasoactive mediators. These mediators are released after IgE receptor cross-linking induced by allergens or other stimuli, including anaphylatoxins (C3a and C5a), aggregated IgG, certain drugs, venoms, and physical stimuli (pressure and temperature changes), as well as cytokines and neuropeptides. The excess release of these mediators can cause variable allergic symptoms and signs, such as bronchospasm, itching, flushing, nausea, vomiting, diarrhea, abdominal pain, vascular instability, and anaphylaxis. Furthermore, mast cell disorders may involve either excessive proliferation of mast cells or abnormal mast cell reactivity. Mast cell disorders can be broadly divided into 3 types: primary, secondary, and idiopathic. All of these disorders present with signs and symptoms of mast cell activation and differ in severity and involvement of various organ systems. The best characterized primary disorder is mastocytosis. Systemic and cutaneous forms of the disease are well described. Secondary disorders include typical allergic diseases and some types of urticarial diseases. In this article, the biochemical characteristics of mast cells and the role of mast cells in allergic inflammation, as well as the classification, diagnosis, and management of mast cell-related disorders, will be reviewed.
Keyword
MeSH Terms
-
Abdominal Pain
Allergens
Allergy and Immunology
Anaphylatoxins
Anaphylaxis
Bronchial Spasm
Classification
Cytokines
Diagnosis
Diarrhea
Flushing
Hypersensitivity
Immunoglobulin E
Immunoglobulin G
Inflammation*
Mast Cells*
Mastocytosis
Nausea
Neuropeptides
Pruritus
Venoms
Vomiting
Allergens
Anaphylatoxins
Cytokines
Immunoglobulin E
Immunoglobulin G
Neuropeptides
Venoms
Figure
Reference
-
1. Rivera J, Fierro NA, Olivera A, Suzuki R. New insights on mast cell activation via the high affinity receptor for IgE. Adv Immunol. 2008; 98:85–120.2. Moon TC, St Laurent CD, Morris KE, Marcet C, Yoshimura T, Sekar Y, et al. Advances in mast cell biology: new understanding of heterogeneity and function. Mucosal Immunol. 2010; 3:111–28.
Article3. Blank U, Falcone FH, Nilsson G. The history of mast cell and basophil research - some lessons learnt from the last century. Allergy. 2013; 68:1093–101.
Article4. Boyce JA. The biology of the mast cell. Allergy Asthma Proc. 2004; 25:27–30.5. Kawakami T, Galli SJ. Regulation of mast-cell and basophil function and survival by IgE. Nat Rev Immunol. 2002; 2:773–86.
Article6. Stone KD, Prussin C, Metcalfe DD. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2010; 125(2 Suppl 2):S73–80.
Article7. Theoharides TC, Alysandratos KD, Angelidou A, Delivanis DA, Sis-manopoulos N, Zhang B, et al. Mast cells and inflammation. Biochim Biophys Acta. 2012; 1822:21–33.
Article8. Boyce JA. Mast cells: beyond IgE. J Allergy Clin Immunol. 2003; 111:24–32.9. Yu Y, Blokhuis BR, Garssen J, Redegeld FA. Non-IgE mediated mast cell activation. Eur J Pharmacol. 2016; 778:33–43.
Article10. Marshall JS, Jawdat DM. Mast cells in innate immunity. J Allergy Clin Immunol. 2004; 114:21–7.
Article11. Saluja R, Khan M, Church MK, Maurer M. The role of IL-33 and mast cells in allergy and inflammation. Clin Transl Allergy. 2015; 5:33.
Article12. Modena BD, Dazy K, White AA. Emerging concepts: mast cell involvement in allergic diseases. Transl Res. 2016; 174:98–121.
Article13. Amin K. The role of mast cells in allergic inflammation. Respir Med. 2012; 106:9–14.
Article14. Serrano-Candelas E, Martinez-Aranguren R, Valero A, Bartra J, Gasta-minza G, Goikoetxea MJ, et al. Comparable actions of omalizumab on mast cells and basophils. Clin Exp Allergy. 2016; 46:92–102.
Article15. Robinson DS. The role of the mast cell in asthma: induction of airway hyperresponsiveness by interaction with smooth muscle? J Allergy Clin Immunol. 2004; 114:58–65.
Article16. Wenzel SE, Fowler AA 3rd, Schwartz LB. Activation of pulmonary mast cells by bronchoalveolar allergen challenge. In vivo release of histamine and tryptase in atopic subjects with and without asthma. Am Rev Respir Dis. 1988; 137:1002–8.17. Church MK, Hiroi J. Inhibition of IgE-dependent histamine release from human dispersed lung mast cells by anti-allergic drugs and salbutamol. Br J Pharmacol. 1987; 90:421–9.
Article18. Boulet LP, Chapman KR, Côté J, Kalra S, Bhagat R, Swystun VA, et al. In-hibitory effects of an anti-IgE antibody E25 on allergen-induced early asthmatic response. Am J Respir Crit Care Med. 1997; 155:1835–40.
Article19. Liu MC, Hubbard WC, Proud D, Stealey BA, Galli SJ, Kagey-Sobotka A, et al. Immediate and late inflammatory responses to ragweed antigen challenge of the peripheral airways in allergic asthmatics. Cellular, mediator, and permeability changes. Am Rev Respir Dis. 1991; 144:51–8.
Article20. Bradding P, Feather IH, Howarth PH, Mueller R, Roberts JA, Britten K, et al. Interleukin 4 is localized to and released by human mast cells. J Exp Med. 1992; 176:1381–6.
Article21. Ying S, Durham SR, Corrigan CJ, Hamid Q, Kay AB. Phenotype of cells expressing mRNA for TH2-type (interleukin 4 and interleukin 5) and TH1-type (interleukin 2 and interferon gamma) cytokines in bronchoalveolar lavage and bronchial biopsies from atopic asthmatic and normal control subjects. Am J Respir Cell Mol Biol. 1995; 12:477–87.
Article22. Humbert M, Grant JA, Taborda-Barata L, Durham SR, Pfister R, Menz G, et al. High-affinity IgE receptor (FcepsilonRI)-bearing cells in bronchial biopsies from atopic and nonatopic asthma. Am J Respir Crit Care Med. 1996; 153(6 Pt 1):1931–7.
Article23. Ying S, Humbert M, Meng Q, Pfister R, Menz G, Gould HJ, et al. Local expression of epsilon germline gene transcripts and RNA for the epsilon heavy chain of IgE in the bronchial mucosa in atopic and nonatopic asthma. J Allergy Clin Immunol. 2001; 107:686–92.24. Humbert M, Durham SR, Ying S, Kimmitt P, Barkans J, Assoufi B, et al. IL-4 and IL-5 mRNA and protein in bronchial biopsies from patients with atopic and nonatopic asthma: evidence against "intrinsic" asthma being a distinct immunopathologic entity. Am J Respir Crit Care Med. 1996; 154:1497–504.
Article25. Del Prete G, Maggi E, Parronchi P, Chrétien I, Tiri A, Macchia D, et al. IL-4 is an essential factor for the IgE synthesis induced in vitro by human T cell clones and their supernatants. J Immunol. 1988; 140:4193–8.26. Barnes PJ. Intrinsic asthma: not so different from allergic asthma but driven by superantigens? Clin Exp Allergy. 2009; 39:1145–51.
Article27. Kwon B, Lee HA, Choi GS, Ye YM, Nahm DH, Park HS. Increased IgG antibody-induced cytotoxicity against airway epithelial cells in patients with nonallergic asthma. J Clin Immunol. 2009; 29:517–23.
Article28. Bentley AM, Jacobson MR, Cumberworth V, Barkans JR, Moqbel R, Schwartz LB, et al. Immunohistology of the nasal mucosa in seasonal allergic rhinitis: increases in activated eosinophils and epithelial mast cells. J Allergy Clin Immunol. 1992; 89:877–83.
Article29. Hanf G, Noga O, O'Connor A, Kunkel G. Omalizumab inhibits allergen challenge-induced nasal response. Eur Respir J. 2004; 23:414–8.
Article30. Saini SS, Paterniti M, Vasagar K, Gibbons SP Jr, Sterba PM, Vonakis BM. Cultured peripheral blood mast cells from chronic idiopathic urticaria patients spontaneously degranulate upon IgE sensitization: relationship to expression of Syk and SHIP-2. Clin Immunol. 2009; 132:342–8.
Article31. Sabroe RA, Greaves MW. Chronic idiopathic urticaria with functional autoantibodies: 12 years on. Br J Dermatol. 2006; 154:813–9.32. Järvikallio A, Naukkarinen A, Harvima IT, Aalto ML, Horsmanheimo M. Quantitative analysis of tryptase- and chymase-containing mast cells in atopic dermatitis and nummular eczema. Br J Dermatol. 1997; 136:871–7.
Article33. Sehra S, Serezani APM, Ocaña JA, Travers JB, Kaplan MH. Mast cells regulate epidermal barrier function and the development of allergic skin inflammation. J Invest Dermatol. 2016; 136:1429–37.34. Schwartz LB. Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol Allergy Clin North Am. 2006; 26:451–63.
Article35. McNeil BD, Pundir P, Meeker S, Han L, Undem BJ, Kulka M, et al. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature. 2015; 519:237–41.
Article36. Schwartz LB, Sakai K, Bradford TR, Ren S, Zweiman B, Worobec AS, et al. The alpha form of human tryptase is the predominant type present in blood at baseline in normal subjects and is elevated in those with systemic mastocytosis. J Clin Invest. 1995; 96:2702–10.
Article37. Schwartz LB, Yunginger JW, Miller J, Bokhari R, Dull D. Time course of appearance and disappearance of human mast cell tryptase in the circulation after anaphylaxis. J Clin Invest. 1989; 83:1551–5.
Article38. Pałgan K, Bartuzi Z. Platelet activating factor in allergies. Int J Immunopathol Pharmacol. 2015; 28:584–9.
Article39. Theoharides TC, Valent P, Akin C. Mast cells, mastocytosis, and related disorders. N Engl J Med. 2015; 373:1885–6.
Article40. Valent P. Mast cell activation syndromes: definition and classification. Allergy. 2013; 68:417–24.
Article41. Valent P, Akin C, Arock M, Brockow K, Butterfield JH, Carter MC, et al. Definitions, criteria and global classification of mast cell disorders with special reference to mast cell activation syndromes: a consensus proposal. Int Arch Allergy Immunol. 2012; 157:215–25.
Article42. Frieri M, Patel R, Celestin J. Mast cell activation syndrome: a review. Curr Allergy Asthma Rep. 2013; 13:27–32.
Article43. Hartmann K, Escribano L, Grattan C, Brockow K, Carter MC, Alvarez-Twose I, et al. Cutaneous manifestations in patients with mastocytosis: Consensus report of the European Competence Network on Mastocytosis; the American Academy of Allergy, Asthma & Immunology; and the European Academy of Allergology and Clinical Immunology. J Allergy Clin Immunol. 2016; 137:35–45.44. Valent P, Akin C, Metcalfe DD. Mastocytosis: 2016 updated WHO classification and novel emerging treatment concepts. Blood. 2017; 129:1420–7.
Article45. Vysniauskaite M, Hertfelder HJ, Oldenburg J, Dreßen P, Brettner S, Homann J, et al. Determination of plasma heparin level improves identi-fication of systemic mast cell activation disease. PLoS One. 2015; 10:e0124912.
Article46. Chang SE, Kang SK, Jee MS, Choi JH, Sung KJ, Moon KC, et al. Clinico-pathological study of 30 Cases of Cutaneous Mastocytosis. Korean J Dermatol. 2002; 40:501–5.47. Park CH, Kim KM, Kim HG, Son SW, Kim SH, Park HW, et al. A case of cutaneous mastocytosis with urticaria developed after hot bath. Korean J Asthma Allergy Clin Immunol. 2005; 25:328–32.48. Lee EH, Kim MR, Kang TW, Kim SC. Diffuse cutaneous mastocytosis with generalized bullae. Ann Dermatol. 2010; 22:77–80.
Article49. Youk J, Koh Y, Kim JW, Kim DY, Park H, Jung WJ, et al. A scientific treatment approach for acute mast cell leukemia: using a strategy based on next-generation sequencing data. Blood Res. 2016; 51:17–22.
Article50. Bae MH, Kim HK, Park CJ, Seo EJ, Park SH, Cho YU, et al. A case of systemic mastocytosis associated with acute myeloid leukemia terminating as aleukemic mast cell leukemia after allogeneic hematopoietic stem cell transplantation. Ann Lab Med. 2013; 33:125–9.
Article51. Seo H, Park SH, Byeon JS, Woo CG, Hong SM, Chang K, et al. Chronic intractable diarrhea caused by gastrointestinal mastocytosis. Intest Res. 2016; 14:280–4.
Article52. Tremblay D, Carreau N, Kremyanskaya M, Mascarenhas J. Systemic mastocytosis: clinical update and future directions. Clin Lymphoma Myelo-ma Leuk. 2015; 15:728–38.
Article53. Hermans MA, Rietveld MJ, van Laar JA, Dalm VA, Verburg M, Pasmans SG, et al. Systemic mastocytosis: a cohort study on clinical characteristics of 136 patients in a large tertiary centre. Eur J Intern Med. 2016; 30:25–30.
Article54. Lim KH, Tefferi A, Lasho TL, Finke C, Patnaik M, Butterfield JH, et al. Systemic mastocytosis in 342 consecutive adults: survival studies and prognostic factors. Blood. 2009; 113:5727–36.
Article55. Molderings GJ, Brettner S, Homann J, Afrin LB. ast cell activation disease: a concise practical guide for diagnostic workup and therapeutic options. J Hematol Oncol. 2011; 4:10.
Article56. Zhang T, Finn DF, Barlow JW, Walsh JJ. Mast cell stabilisers. Eur J Pharmacol. 2016; 778:158–68.
Article57. Chang TW, Chen C, Lin CJ, Metz M, Church MK, Maurer M. The potential pharmacologic mechanisms of omalizumab in patients with chronic spontaneous urticaria. J Allergy Clin Immunol. 2015; 135:337–42.
Article58. Metz M, Ohanyan T, Church MK, Maurer M. Omalizumab is an effective and rapidly acting therapy in difficult-to-treat chronic urticaria: a retrospective clinical analysis. J Dermatol Sci. 2014; 73:57–62.
Article59. Bell MC, Jackson DJ. Prevention of anaphylaxis related to mast cell activation syndrome with omalizumab. Ann Allergy Asthma Immunol. 2012; 108:383–4.
Article60. Jagdis A, Vadas P. Omalizumab effectively prevents recurrent refractory anaphylaxis in a patient with monoclonal mast cell activation syndrome. Ann Allergy Asthma Immunol. 2014; 113:115–6.
Article61. Akin C, Fumo G, Yavuz AS, Lipsky PE, Neckers L, Metcalfe DD. A novel form of mastocytosis associated with a transmembrane c-kit mutation and response to imatinib. Blood. 2004; 103:3222–5.
Article62. Cahill KN, Katz HR, Cui J, Lai J, Kazani S, Crosby-Thompson A, et al. KIT inhibition by imatinib in patients with severe refractory asthma. N Engl J Med. 2017; 376:1911–20.
Article63. Krauth MT, Mirkina I, Herrmann H, Baumgartner C, Kneidinger M, Valent P. Midostaurin (PKC412) inhibits immunoglobulin E-dependent activation and mediator release in human blood basophils and mast cells. Clin Exp Allergy. 2009; 39:1711–20.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Mast Cells and Allergic Rhinitis
- Experimental Study on The Role of Various Antihistaminics to Tissue Mast Cell Changes Elicited by Ultraviolet Ray Inflammation
- Role of Mast Cells in Allergic Inflammation and Innate Immunity
- Prostaglandin D2 and TH2 Inflammation in the Pathogenesis of Bronchial Asthma
- The Role of Cytokines in Allergy