Clin Exp Vaccine Res.
2013 Jan;2(1):58-65.
Inactivated pep27 mutant as an effective mucosal vaccine against a secondary lethal pneumococcal challenge in mice
- Affiliations
-
- 1Sungkyunkwan University School of Pharmacy, Suwon, Korea. dkrhee@skku.edu
- 2Department of Microbiology, University of Alabama at Birmingham, Birmingham, AL, USA.
Abstract
- PURPOSE
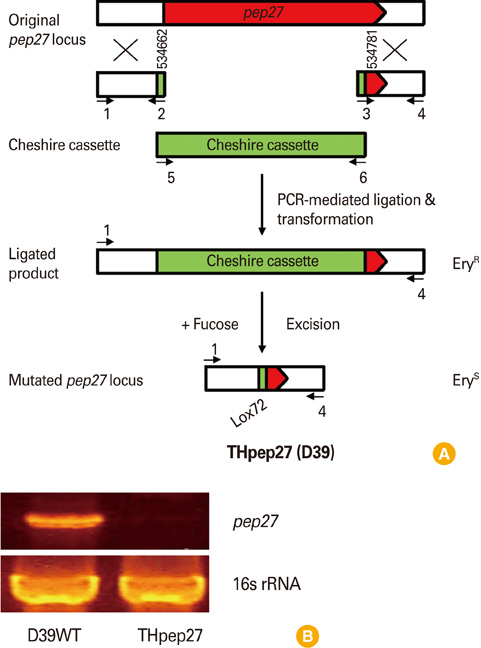
A pep27 mutant may be able to elicit mucosal immunity against pneumococcal diseases, and could be employed as an inexpensive attenuated vaccine. However, this particular mutant contains an erythromycin-resistance marker. The purpose of the current study is to develop a markerless pep27 mutant and assess whether this inactivated mutant is able to induce mucosal immunity.
MATERIALS AND METHODS
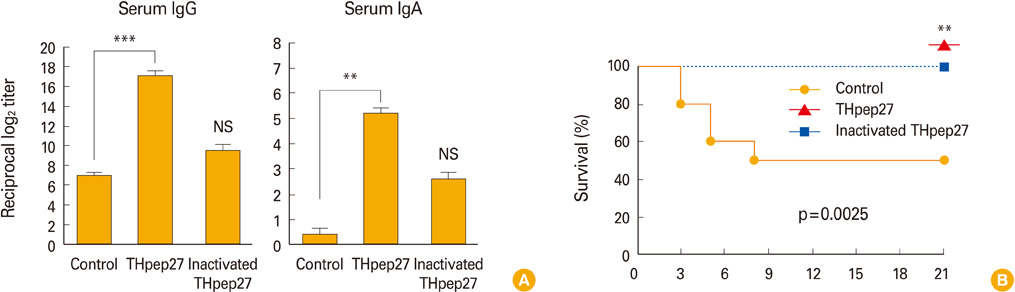
Mice were vaccinated intranasally with the inactivated markerless pep27 mutant every 2 weeks for a total of three times, after which time serum samples were analyzed for antibody titers. The mice were then challenged with a lethal D39 strain and their survival time was measured. The cross-reactivity of the antisera against pep27 was also compared to other mutant serotypes.
RESULTS
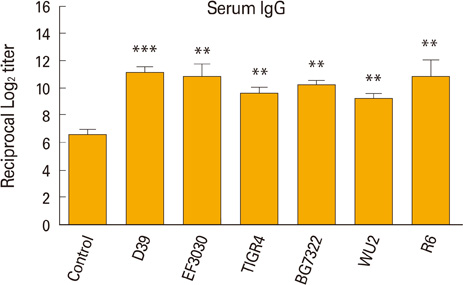
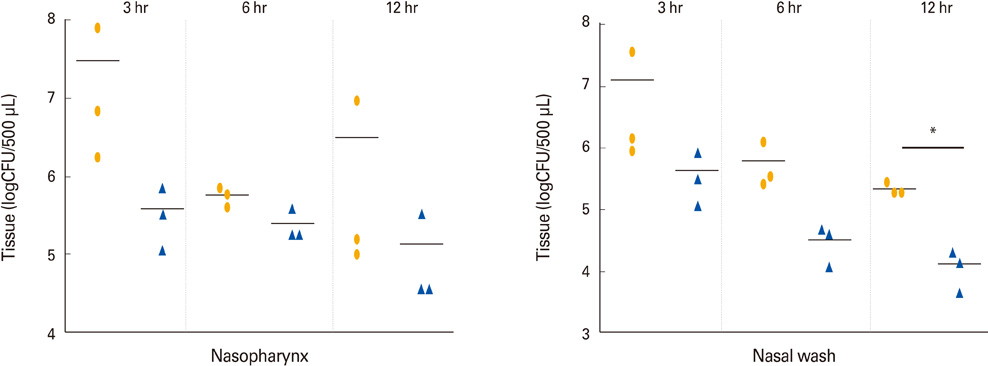
Intranasal immunization of mice with the inactivated markerless pep27 mutant provides effective protection and rapidly cleared bacterial colonization in vivo. Moreover, antisera raised against the pep27 mutant may cross-react with several other serotype strains.
CONCLUSION
Intranasal immunization with the inactivated pep27 mutant may be able to provide mucosal immunity, and could represent an efficient mucosal vaccine.
Keyword
MeSH Terms
Figure
Reference
-
1. Paton JC. The contribution of pneumolysin to the pathogenicity of Streptococcus pneumoniae. Trends Microbiol. 1996. 4:103–106.
Article2. Paton JC, Lock RA, Lee CJ, et al. Purification and immunogenicity of genetically obtained pneumolysin toxoids and their conjugation to Streptococcus pneumoniae type 19F polysaccharide. Infect Immun. 1991. 59:2297–2304.
Article3. Gray BM, Converse GM 3rd, Dillon HC Jr. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J Infect Dis. 1980. 142:923–933.
Article4. Park IH, Pritchard DG, Cartee R, Brandao A, Brandileone MC, Nahm MH. Discovery of a new capsular serotype (6C) within serogroup 6 of Streptococcus pneumoniae. J Clin Microbiol. 2007. 45:1225–1233.
Article5. Bogaert D, Hermans PW, Adrian PV, Rumke HC, de Groot R. Pneumococcal vaccines: an update on current strategies. Vaccine. 2004. 22:2209–2220.
Article6. Isaacman DJ, McIntosh ED, Reinert RR. Burden of invasive pneumococcal disease and serotype distribution among Streptococcus pneumoniae isolates in young children in Europe: impact of the 7-valent pneumococcal conjugate vaccine and considerations for future conjugate vaccines. Int J Infect Dis. 2010. 14:e197–e209.
Article7. Oosterhuis-Kafeja F, Beutels P, Van Damme P. Immunogenicity, efficacy, safety and effectiveness of pneumococcal conjugate vaccines (1998-2006). Vaccine. 2007. 25:2194–2212.
Article8. Poolman JT. Pneumococcal vaccine development. Expert Rev Vaccines. 2004. 3:597–604.
Article9. Jambo KC, Sepako E, Heyderman RS, Gordon SB. Potential role for mucosally active vaccines against pneumococcal pneumonia. Trends Microbiol. 2010. 18:81–89.
Article10. Kayhty H, Auranen K, Nohynek H, Dagan R, Makela H. Nasopharyngeal colonization: a target for pneumococcal vaccination. Expert Rev Vaccines. 2006. 5:651–667.
Article11. Brandtzaeg P. Molecular and cellular aspects of the secretory immunoglobulin system. APMIS. 1995. 103:1–19.
Article12. Aaberge IS, Groeng EC, Haugen IL, Dalseg R, Lovik M, Haneberg B. Induction of systemic and mucosal antibodies to pneumococcal antigens by mucosal immunization. J Cell Biochem Suppl. 1995. 19A:242.13. Hvalbye BK, Aaberge IS, Lovik M, Haneberg B. Intranasal immunization with heat-inactivated Streptococcus pneumoniae protects mice against systemic pneumococcal infection. Infect Immun. 1999. 67:4320–4325.
Article14. McEllistrem MC, Adams J, Mason EO, Wald ER. Epidemiology of acute otitis media caused by Streptococcus pneumoniae before and after licensure of the 7-valent pneumococcal protein conjugate vaccine. J Infect Dis. 2003. 188:1679–1684.
Article15. Oliveira ML, Areas AP, Ho PL. Intranasal vaccines for protection against respiratory and systemic bacterial infections. Expert Rev Vaccines. 2007. 6:419–429.
Article16. Kim EH, Choi SY, Kwon MK, et al. Streptococcus pneumoniae pep27 mutant as a live vaccine for serotype-independent protection in mice. Vaccine. 2012. 30:2008–2019.
Article17. Avery OT, Macleod CM, McCarty M. Studies on the chemical nature of the substance inducing transformation of pneumococcal types: induction of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type III. J Exp Med. 1944. 79:137–158.18. Ottolenghi E, Hotchkiss RD. Release of genetic transforming agent from pneumococcal cultures during growth and disintegration. J Exp Med. 1962. 116:491–519.
Article19. Briles DE, Nahm M, Schroer K, et al. Antiphosphocholine antibodies found in normal mouse serum are protective against intravenous infection with type 3 Streptococcus pneumoniae. J Exp Med. 1981. 153:694–705.
Article20. Aaberge IS, Eng J, Lermark G, Lovik M. Virulence of Streptococcus pneumoniae in mice: a standardized method for preparation and frozen storage of the experimental bacterial inoculum. Microb Pathog. 1995. 18:141–152.
Article21. Briles DE, Crain MJ, Gray BM, Forman C, Yother J. Strong association between capsular type and virulence for mice among human isolates of Streptococcus pneumoniae. Infect Immun. 1992. 60:111–116.
Article22. Andersson B, Dahmen J, Frejd T, et al. Identification of an active disaccharide unit of a glycoconjugate receptor for pneumococci attaching to human pharyngeal epithelial cells. J Exp Med. 1983. 158:559–570.
Article23. Weng L, Biswas I, Morrison DA. A self-deleting Cre-lox-ermAM cassette, Cheshire, for marker-less gene deletion in Streptococcus pneumoniae. J Microbiol Methods. 2009. 79:353–357.
Article24. Havarstein LS, Coomaraswamy G, Morrison DA. An unmodified heptadecapeptide pheromone induces competence for genetic transformation in Streptococcus pneumoniae. Proc Natl Acad Sci U S A. 1995. 92:11140–11144.
Article25. Bricker AL, Camilli A. Transformation of a type 4 encapsulated strain of Streptococcus pneumoniae. FEMS Microbiol Lett. 1999. 172:131–135.
Article26. Kwon HY, Kim SW, Choi MH, et al. Effect of heat shock and mutations in ClpL and ClpP on virulence gene expression in Streptococcus pneumoniae. Infect Immun. 2003. 71:3757–3765.
Article27. Roche AM, Weiser JN. Identification of the targets of cross-reative antibodies induced by Streptococcus pneumoniae colonization. Infect Immun. 2010. 78:2231–2239.
Article28. Roche AM, King SJ, Weiser JN. Live attenuated Streptococcus pneumoniae strains induce serotype-independent mucosal and systemic protection in mice. Infect Immun. 2007. 75:2469–2475.
Article29. Pneumococcal vaccines. WHO position paper. Wkly Epidemiol Rec. 1999. 74:177–183.30. Tyrrell GJ, Lovgren M, Chui N, et al. Serotypes and antimicrobial susceptibilities of invasive Streptococcus pneumoniae pre- and post-seven valent pneumococcal conjugate vaccine introduction in Alberta, Canada, 2000-2006. Vaccine. 2009. 27:3553–3560.
Article31. Gladstone RA, Jefferies JM, Faust SN, Clarke SC. Continued control of pneumococcal disease in the UK: the impact of vaccination. J Med Microbiol. 2011. 60(Pt 1):1–8.
Article32. Malley R, Lipsitch M, Stack A, et al. Intranasal immunization with killed unencapsulated whole cells prevents colonization and invasive disease by capsulated pneumococci. Infect Immun. 2001. 69:4870–4873.
Article33. Mutsch M, Zhou W, Rhodes P, et al. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N Engl J Med. 2004. 350:896–903.
Article34. Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983. 65:55–63.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy and effectiveness of extended-valency pneumococcal conjugate vaccines
- Invasive Pneumococcal Diseases in Korean Adults After the Introduction of Pneumococcal Vaccine into the National Immunization Program
- Inactivated genotype 1 Japanese encephalitis vaccine for swine
- Pneumococcal Vaccine
- Development of inactivated Akabane and bovine ephemeral fever vaccine for cattle