Obstet Gynecol Sci.
2016 Sep;59(5):357-366. 10.5468/ogs.2016.59.5.357.
Screening for chromosomal abnormalities using combined test in the first trimester of pregnancy
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Ewha Womans University School of Medicine, Seoul, Korea. ewhapmh@ewha.ac.kr
- 2Kwak Women's Hospital, Seongnam, Korea.
- KMID: 2391685
- DOI: http://doi.org/10.5468/ogs.2016.59.5.357
Abstract
OBJECTIVE
This study was designed to review the screening performance of combined test at the Ewha Womans University Mokdong hospital.
METHODS
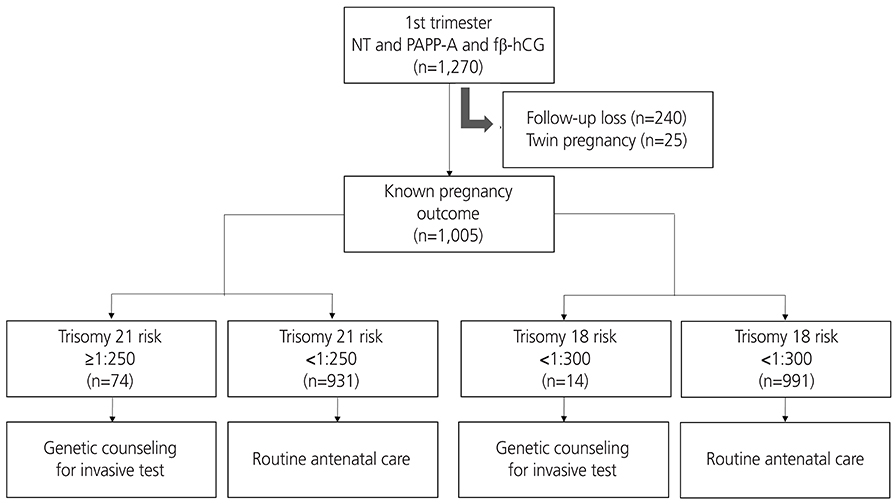
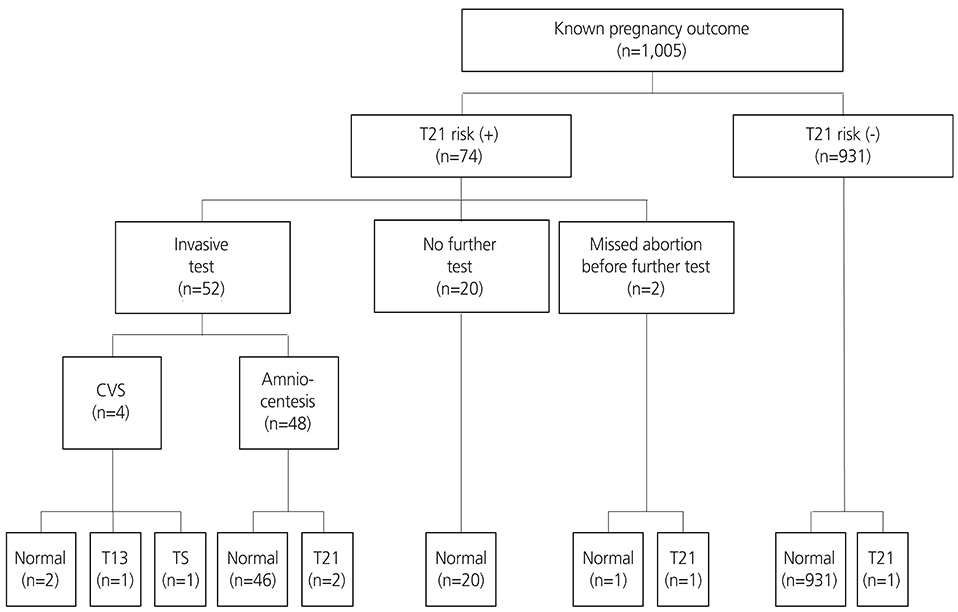
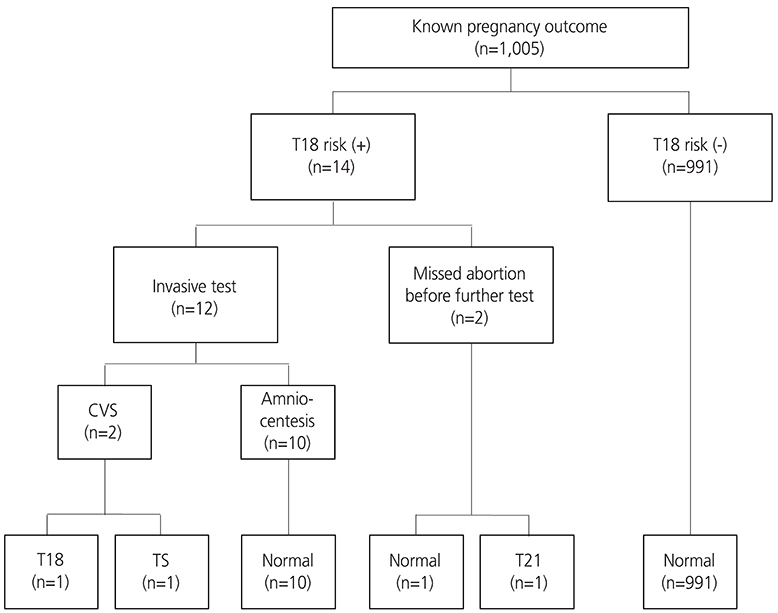
All women admitted for routine antenatal care between January 1st 2008 and December 31st 2012 with a known pregnancy outcome were included in this study, totaling 1,156 women with singleton pregnancies presenting at 10 to 13 weeks of gestation. Women were offered screening using a combination of maternal serum pregnancy-associated plasma protein-A, free β-human chorionic gonadotropin and fetal nuchal translucency thickness. Those with an estimated risk of ≥1 in 250 of carrying a fetus with trisomy 21 or ≥1 in 300 risk of trisomy 18 were offered genetic counseling with the option of an invasive diagnostic test.
RESULTS
The median of gestational age was 11+3 weeks, the median of crown-rump length was 47.1 mm, and the median age of the women was 31 years. The detection rate was 80% for trisomy 21 (4 of 5) and 100% for trisomy 13 and 18 (all 2). The false-positive rate was 7.73% for trisomy 21 and 1.21% for trisomy 18.
CONCLUSION
This study was the first large population study performed with the aim of analyzing the performance of the combined test in Korea. This study demonstrated that the detection rates and other figures of the first trimester combined test are comparable to the results reported in other papers worldwide. Consequently, if strict conditions for good screening outcomes are achieved, the first trimester combined test might well be the earliest detectable screening, improving detection rates without increasing karyotyping or economic and other implications that inevitably ensue.
Keyword
MeSH Terms
-
Chorionic Gonadotropin
Chromosome Aberrations*
Crown-Rump Length
Diagnostic Tests, Routine
Down Syndrome
Female
Fetus
Genetic Counseling
Gestational Age
Humans
Korea
Mass Screening*
Nuchal Translucency Measurement
Pregnancy
Pregnancy Outcome
Pregnancy Trimester, First*
Pregnancy*
Pregnancy-Associated Plasma Protein-A
Prenatal Diagnosis
Trisomy
Chorionic Gonadotropin
Pregnancy-Associated Plasma Protein-A
Figure
Reference
-
1. ACOG Committee on Practice Bulletins. ACOG Practice Bulletin No. 77: screening for fetal chromosomal abnormalities. Obstet Gynecol. 2007; 109:217–227.2. Schuchter K, Hafner E, Stangl G, Metzenbauer M, Hofinger D, Philipp K. The first trimester 'combined test' for the detection of Down syndrome pregnancies in 4939 unselected pregnancies. Prenat Diagn. 2002; 22:211–215.3. Wald NJ, Hackshaw AK. Combining ultrasound and biochemistry in first-trimester screening for Down's syndrome. Prenat Diagn. 1997; 17:821–829.4. Spencer K, Spencer CE, Power M, Moakes A, Nicolaides KH. One stop clinic for assessment of risk for fetal anomalies: a report of the first year of prospective screening for chromosomal anomalies in the first trimester. BJOG. 2000; 107:1271–1275.5. Spencer K, Spencer CE, Power M, Dawson C, Nicolaides KH. Screening for chromosomal abnormalities in the first trimester using ultrasound and maternal serum biochemistry in a one-stop clinic: a review of three years prospective experience. BJOG. 2003; 110:281–286.6. Nicolaides KH, Spencer K, Avgidou K, Faiola S, Falcon O. Multicenter study of first-trimester screening for trisomy 21 in 75 821 pregnancies: results and estimation of the potential impact of individual risk-orientated two-stage first-trimester screening. Ultrasound Obstet Gynecol. 2005; 25:221–226.7. Wald NJ, Rodeck C, Hackshaw AK, Rudnicka A. SURUSS in perspective. Semin Perinatol. 2005; 29:225–235.8. Kagan KO, Wright D, Baker A, Sahota D, Nicolaides KH. Screening for trisomy 21 by maternal age, fetal nuchal translucency thickness, free beta-human chorionic gonadotropin and pregnancy-associated plasma protein-A. Ultrasound Obstet Gynecol. 2008; 31:618–624.9. Wald NJ, Rodeck C, Hackshaw AK, Walters J, Chitty L, Mackinson AM, et al. First and second trimester antenatal screening for Down's syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS). Health Technol Assess. 2003; 7:1–77.10. Engels MA, Heijboer AC, Blankenstein MA, van Vugt JM. Performance of first-trimester combined test for Down syndrome in different maternal age groups: reason for adjustments in screening policy? Prenat Diagn. 2011; 31:1241–1245.11. De Biasio P, Siccardi M, Volpe G, Famularo L, Santi F, Canini S. First-trimester screening for Down syndrome using nuchal translucency measurement with free beta-hCG and PAPP-A between 10 and 13 weeks of pregnancy: the combined test. Prenat Diagn. 1999; 19:360–363.12. Crossley JA, Aitken DA, Cameron AD, McBride E, Connor JM. Combined ultrasound and biochemical screening for Down's syndrome in the first trimester: a Scottish multicentre study. BJOG. 2002; 109:667–676.13. Lee FK, Chen LC, Cheong ML, Chou CY, Tsai MS. First trimester combined test for Down syndrome screening in unselected pregnancies: a report of a 13-year experience. Taiwan J Obstet Gynecol. 2013; 52:523–526.14. Wald NJ, Cuckle HS, Densem JW, Nanchahal K, Royston P, Chard T, et al. Maternal serum screening for Down's syndrome in early pregnancy. BMJ. 1988; 297:883–887.15. Cuckle H. Biochemical screening for Down syndrome. Eur J Obstet Gynecol Reprod Biol. 2000; 92:97–101.16. Baer RJ, Flessel MC, Jelliffe-Pawlowski LL, Goldman S, Hudgins L, Hull AD, et al. Detection rates for aneuploidy by first-trimester and sequential screening. Obstet Gynecol. 2015; 126:753–759.17. Tul N, Spencer K, Noble P, Chan C, Nicolaides K. Screening for trisomy 18 by fetal nuchal translucency and maternal serum free beta-hCG and PAPP-A at 10-14 weeks of gestation. Prenat Diagn. 1999; 19:1035–1042.18. Sherod C, Sebire NJ, Soares W, Snijders RJ, Nicolaides KH. Prenatal diagnosis of trisomy 18 at the 10-14-week ultrasound scan. Ultrasound Obstet Gynecol. 1997; 10:387–390.19. Spencer K, Mallard AS, Coombes EJ, Macri JN. Prenatal screening for trisomy 18 with free beta human chorionic gonadotrophin as a marker. BMJ. 1993; 307:1455–1458.20. Biagiotti R, Cariati E, Brizzi L, Cappelli G, D'Agata A. Maternal serum screening for trisomy 18 in the first trimester of pregnancy. Prenat Diagn. 1998; 18:907–913.21. Schuchter K, Wald N, Hackshaw AK, Hafner E, Liebhart E. The distribution of nuchal translucency at 10-13 weeks of pregnancy. Prenat Diagn. 1998; 18:281–286.22. Malone FD, Canick JA, Ball RH, Nyberg DA, Comstock CH, Bukowski R, et al. First-trimester or second-trimester screening, or both, for Down's syndrome. N Engl J Med. 2005; 353:2001–2011.23. Choi YK, Kim MY, Han JY, Ryu HM, Yang JH, Kim ES, et al. A study about the effectiveness of triple marker test as a screening test for chromosomal aneuploidy. Korean J Obstet Gynecol. 1999; 42:1935–1942.24. Wald NJ, Rodeck C, Hackshaw AK, Walters J, Chitty L, Mackinson AM. First and second trimester antenatal screening for Down's syndrome: the results of the Serum, Urine and Ultrasound Screening Study (SURUSS). J Med Screen. 2003; 10:56–104.25. Alfirevic Z, Sundberg K, Brigham S. Amniocentesis and chorionic villus sampling for prenatal diagnosis. Cochrane Database Syst Rev. 2003; (3):CD003252.26. Wald NJ, Rodeck C, Hackshaw AK, Rudnicka A. SURUSS in perspective. BJOG. 2004; 111:521–531.27. Gyselaers WJ, Vereecken AJ, Van Herck EJ, Straetmans DP, de Jonge ET, Ombelet WU, et al. Population screening for fetal trisomy 21: easy access to screening should be balanced against a uniform ultrasound protocol. Prenat Diagn. 2005; 25:984–990.28. Nicolaides KH, Azar G, Byrne D, Mansur C, Marks K. Fetal nuchal translucency: ultrasound screening for chromosomal defects in first trimester of pregnancy. BMJ. 1992; 304:867–869.29. Sebire NJ, Spencer K, Noble PL, Hughes K, Nicolaides KH. Maternal serum alpha-fetoprotein in fetal neural tube and abdominal wall defects at 10 to 14 weeks of gestation. Br J Obstet Gynaecol. 1997; 104:849–851.30. Johnson SP, Sebire NJ, Snijders RJ, Tunkel S, Nicolaides KH. Ultrasound screening for anencephaly at 10-14 weeks of gestation. Ultrasound Obstet Gynecol. 1997; 9:14–16.31. Campbell J, Gilbert WM, Nicolaides KH, Campbell S. Ultrasound screening for spina bifida: cranial and cerebellar signs in a high-risk population. Obstet Gynecol. 1987; 70:247–250.32. Gil MM, Revello R, Poon LC, Akolekar R, Nicolaides KH. Clinical implementation of routine screening for fetal trisomies in the UK NHS: cell-free DNA test contingent on results from first-trimester combined test. Ultrasound Obstet Gynecol. 2016; 47:45–52.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Fetal Sonography for the Detection of Chromosomal Abnormality
- The efficacy of nuchal translucency with free beta-hCG, PAPP-A as a screening test for detection of chromosomal anomaly in the first trimester of pregnancy
- Recent Trends in Prenatal Diagnosis of Fetal Malformations
- Fetal Nuchal Translucency Measurement for Detection of Chromosomal Abnormalities in the First Trimester of High Risk Pregnancy
- Screening ultrasonography in pregnancy