J Gastric Cancer.
2015 Dec;15(4):290-294. 10.5230/jgc.2015.15.4.290.
C-Kit-Negative Gastrointestinal Stromal Tumor in the Stomach
- Affiliations
-
- 1Division of Gastrointestinal Surgery, Department of Surgery, The Catholic University of Korea, Seoul, Korea. painkiller9@catholic.ac.kr
- 2Department of Hospital Pathology, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 2391565
- DOI: http://doi.org/10.5230/jgc.2015.15.4.290
Abstract
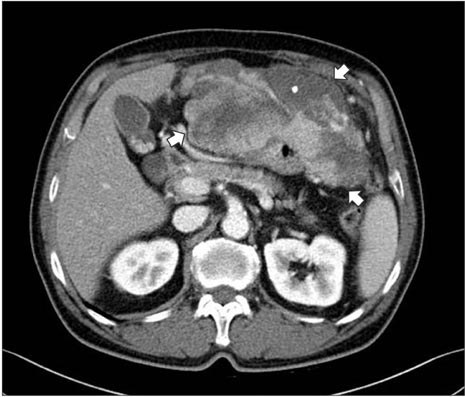
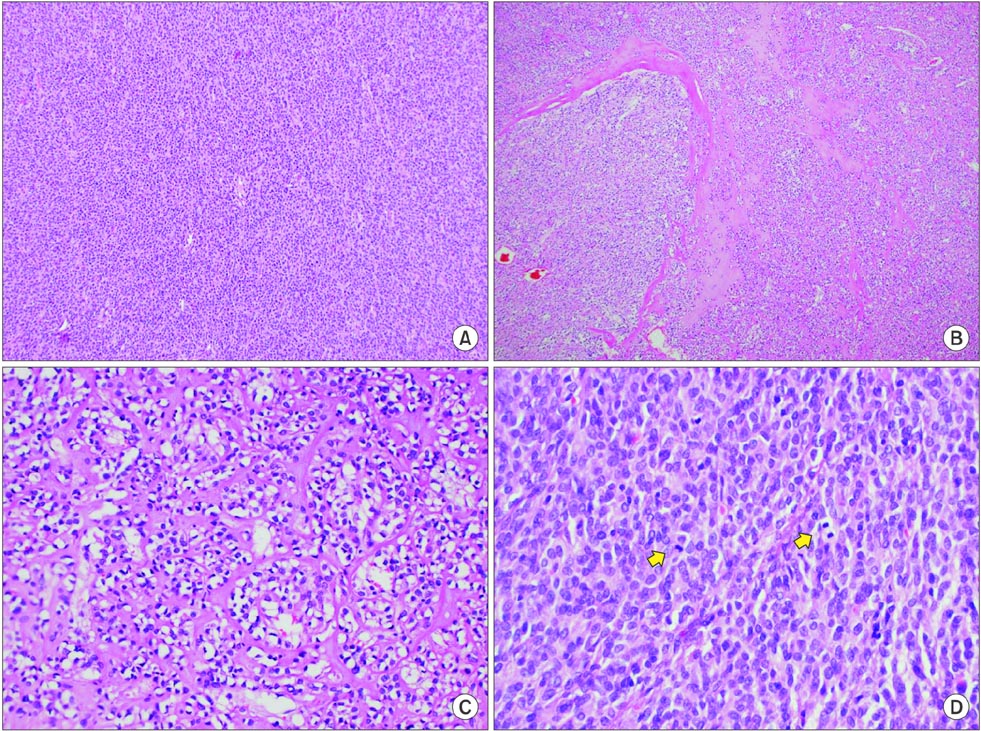
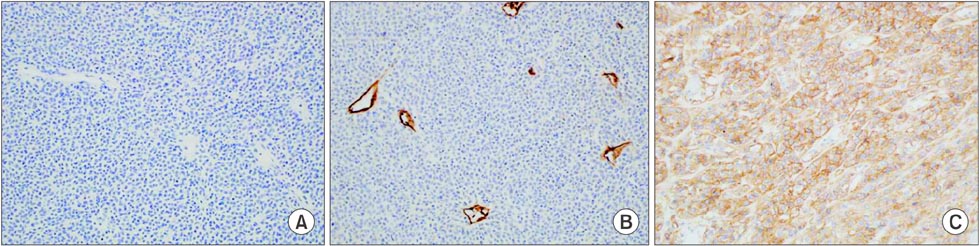
- C-kit-negative gastrointestinal stromal tumors (GISTs) are uncommon, and there have been few reports about the diagnosis and treatment of c-kit-negative GISTs in the stomach. We report the case of a patient who was diagnosed with a huge and atypical GIST in the stomach. The GIST was completely resected and finally diagnosed as c-kit-negative GIST based on immunohistochemical staining of tumor cells, which were negative for CD117 and CD34 and positive for Discovered on GIST-1 (DOG1). C-kit-negative GISTs could be treated by complete resection and/or imatinib, which is the same treatment for c-kit-positive GISTs.
MeSH Terms
Figure
Reference
-
1. Ducimetière F, Lurkin A, Ranchère-Vince D, Decouvelaere AV, Pèoc'h M, Istier L, et al. Incidence of sarcoma histotypes and molecular subtypes in a prospective epidemiological study with central pathology review and molecular testing. PLoS One. 2011; 6:e20294.2. Nilsson B, Bümming P, Meis-Kindblom JM, Odèn A, Dortok A, Gustavsson B, et al. Gastrointestinal stromal tumors: the incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era: a population-based study in western Sweden. Cancer. 2005; 103:821–829.3. Tran T, Davila JA, El-Serag HB. The epidemiology of malignant gastrointestinal stromal tumors: an analysis of 1,458 cases from 1992 to 2000. Am J Gastroenterol. 2005; 100:162–168.4. Cho MY, Sohn JH, Kim JM, Kim KM, Park YS, Kim WH, et al. Current trends in the epidemiological and pathological characteristics of gastrointestinal stromal tumors in Korea, 2003-2004. J Korean Med Sci. 2010; 25:853–862.5. Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998; 279:577–580.6. Corless CL, Heinrich MC. Molecular pathobiology of gastrointestinal stromal sarcomas. Annu Rev Pathol. 2008; 3:557–586.7. Medeiros F, Corless CL, Duensing A, Hornick JL, Oliveira AM, Heinrich MC, et al. KIT-negative gastrointestinal stromal tumors: proof of concept and therapeutic implications. Am J Surg Pathol. 2004; 28:889–894.8. Joensuu H, Hohenberger P, Corless CL. Gastrointestinal stromal tumour. Lancet. 2013; 382:973–983.9. Kang GH, Srivastava A, Kim YE, Park HJ, Park CK, Sohn TS, et al. DOG1 and PKC-θ are useful in the diagnosis of KIT negative gastrointestinal stromal tumors. Mod Pathol. 2011; 24:866–875.10. Espinosa I, Lee CH, Kim MK, Rouse BT, Subramanian S, Montgomery K, et al. A novel monoclonal antibody against DOG1 is a sensitive and specific marker for gastrointestinal stromal tumors. Am J Surg Pathol. 2008; 32:210–218.11. Liegl B, Hornick JL, Corless CL, Fletcher CD. Monoclonal antibody DOG1.1 shows higher sensitivity than KIT in the diagnosis of gastrointestinal stromal tumors, including unusual subtypes. Am J Surg Pathol. 2009; 33:437–446.12. Miettinen M, Wang ZF, Lasota J. DOG1 antibody in the differential diagnosis of gastrointestinal stromal tumors: a study of 1840 cases. Am J Surg Pathol. 2009; 33:1401–1408.13. Hohenberger P, Ronellenfitsch U, Oladeji O, Pink D, Ströbel P, Wardelmann E, et al. Pattern of recurrence in patients with ruptured primary gastrointestinal stromal tumour. Br J Surg. 2010; 97:1854–1859.14. Deshaies I, Cherenfant J, Gusani NJ, Jiang Y, Harvey HA, Kimchi ET, et al. Gastrointestinal stromal tumor (GIST) recurrence following surgery: review of the clinical utility of imatinib treatment. Ther Clin Risk Manag. 2010; 6:453–458.15. Blanke CD, Rankin C, Demetri GD, Ryan CW, von Mehren M, Benjamin RS, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol. 2008; 26:626–632.16. Dematteo RP, Ballman KV, Antonescu CR, Maki RG, Pisters PW, Demetri GD, et al. American College of Surgeons Oncology Group (ACOSOG) Intergroup Adjuvant GIST Study Team. Adjuvant imatinib mesylate after resection of localised, primary gastrointestinal stromal tumour: a randomised, doubleblind, placebo-controlled trial. Lancet. 2009; 373:1097–1104.17. DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: recurrence patterns and prognostic factors for survival. Ann Surg. 2000; 231:51–58.18. West RB, Corless CL, Chen X, Rubin BP, Subramanian S, Montgomery K, et al. The novel marker, DOG1, is expressed ubiquitously in gastrointestinal stromal tumors irrespective of KIT or PDGFRA mutation status. Am J Pathol. 2004; 165:107–113.19. Dewar AL, Cambareri AC, Zannettino AC, Miller BL, Doherty KV, Hughes TP, et al. Macrophage colony-stimulating factor receptor c-fms is a novel target of imatinib. Blood. 2005; 105:3127–3132.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- KIT-negative Gastrointestinal Stromal Tumor in a Child: A Case Report
- Gastrointestinal Stromal Tumor of the Rectum in a Pediatric
- Carney Triad in an Adult with Aggressive Behavior: The First Case in Korea
- Gastrointestinal stromal tumor with KIT mutation in neurofibromatosis type 1
- A case of huge Gastrointestinal stromal tumor masquerading as an ovarian malignancy