J Gastric Cancer.
2015 Dec;15(4):238-245. 10.5230/jgc.2015.15.4.238.
Time-Dependent Effects of Prognostic Factors in Advanced Gastric Cancer Patients
- Affiliations
-
- 1Department of Surgery, Korea Cancer Center Hospital, Korea Institute of Radiological and Medical Sciences, Seoul, Korea. shjin@kcch.re.kr
- 2Department of Surgery, Dongnam Institute of Radiological and Medical Sciences, Busan, Korea.
- 3Department of Pathology, Dongnam Institute of Radiological and Medical Sciences, Busan, Korea.
- 4Department of Thoracic Surgery, Korea Cancer Center Hospital, Korea Institute of Radiological and Medical Sciences, Seoul, Korea.
- 5Department of Pathology, Korea Cancer Center Hospital, Korea Institute of Radiological and Medical Sciences, Seoul, Korea.
- 6Department of Surgery, Konkuk University School of Medicine, Seoul, Korea.
- KMID: 2391558
- DOI: http://doi.org/10.5230/jgc.2015.15.4.238
Abstract
- PURPOSE
This study aimed to identify time-dependent prognostic factors and demonstrate the time-dependent effects of important prognostic factors in patients with advanced gastric cancer (AGC).
MATERIALS AND METHODS
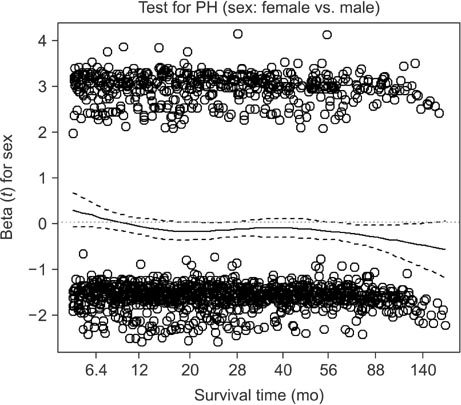
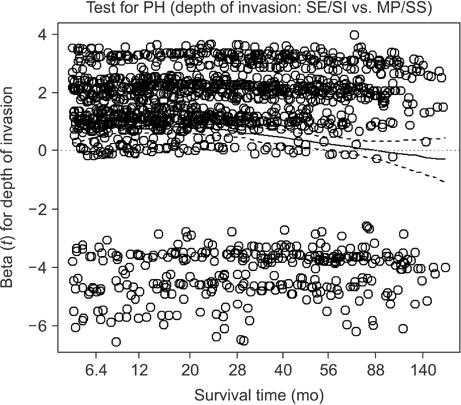
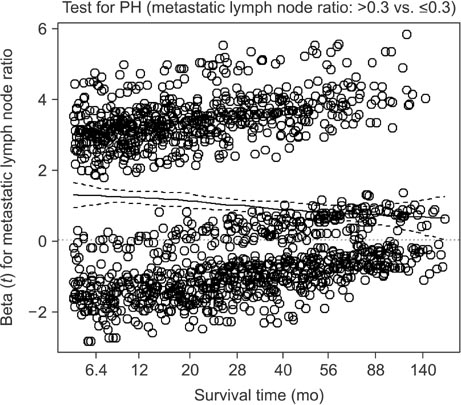
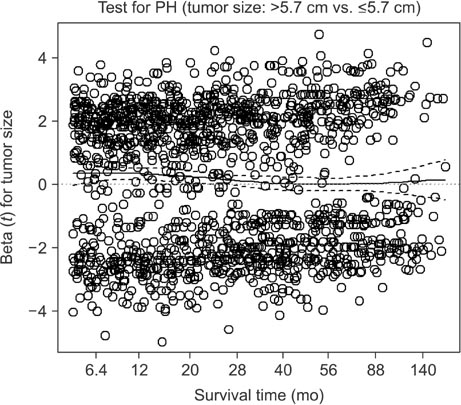
We retrospectively evaluated 3,653 patients with AGC who underwent curative standard gastrectomy between 1991 and 2005 at the Korea Cancer Center Hospital. Multivariate survival analysis with Cox proportional hazards regression was used in the analysis. A non-proportionality test based on the Schoenfeld residuals (also known as partial residuals) was performed, and scaled Schoenfeld residuals were plotted over time for each covariate.
RESULTS
The multivariate analysis revealed that sex, depth of invasion, metastatic lymph node (LN) ratio, tumor size, and chemotherapy were time-dependent covariates violating the proportional hazards assumption. The prognostic effects (i.e., log of hazard ratio [LHR]) of the time-dependent covariates changed over time during follow-up, and the effects generally diminished with low slope (e.g., depth of invasion and tumor size), with gentle slope (e.g., metastatic LN ratio), or with steep slope (e.g., chemotherapy). Meanwhile, the LHR functions of some covariates (e.g., sex) crossed the zero reference line from positive (i.e., bad prognosis) to negative (i.e., good prognosis).
CONCLUSIONS
The time-dependent effects of the prognostic factors of AGC are clearly demonstrated in this study. We can suggest that time-dependent effects are not an uncommon phenomenon among prognostic factors of AGC.
Keyword
MeSH Terms
Figure
Reference
-
1. Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM classification of malignant tumours. New York: John Wiley & Sons;2011.2. Gospodarowicz M, O'Sullivan B. Prognostic factors in cancer. Semin Surg Oncol. 2003; 21:13–18.3. Cox DR. Regression models and life-tables. J R Stat Soc Series B Stat Methodol. 1972; 34:187–220.4. Bradburn MJ, Clark TG, Love SB, Altman DG. Survival analysis part II: multivariate data analysis: an introduction to concepts and methods. Br J Cancer. 2003; 89:431–436.5. Ahmed FE, Vos PW, Holbert D. Modeling survival in colon cancer: a methodological review. Mol Cancer. 2007; 6:15.6. Hilsenbeck SG, Ravdin PM, de Moor CA, Chamness GC, Osborne CK, Clark GM. Time-dependence of hazard ratios for prognostic factors in primary breast cancer. In : Gasparini G, editor. Prognostic variables in node-negative and node-positive breast cancer. Boston, MA: Springer;1998. p. 317–327.7. Bellera CA, MacGrogan G, Debled M, de Lara CT, Brouste V, Mathoulin-Pélissier S. Variables with time-varying effects and the Cox model: some statistical concepts illustrated with a prognostic factor study in breast cancer. BMC Med Res Methodol. 2010; 10:20.8. Warwick J, Tabàr L, Vitak B, Duffy SW. Time-dependent effects on survival in breast carcinoma: results of 20 years of follow-up from the Swedish two-county study. Cancer. 2004; 100:1331–1336.9. Kattan MW, Karpeh MS, Mazumdar M, Brennan MF. Postoperative nomogram for disease-specific survival after an R0 resection for gastric carcinoma. J Clin Oncol. 2003; 21:3647–3650.10. Eom BW, Ryu KW, Nam BH, Park Y, Lee HJ, Kim MC, et al. Survival nomogram for curatively resected Korean gastric cancer patients: multicenter retrospective analysis with external validation. PLoS One. 2015; 10:e0119671.11. Park JI, Jin SH, Bang HY, Paik NS, Moon NM, Lee JI. Survival rates after operation for gastric cancer: fifteen-year experience at a Korea Cancer Center Hospital. J Korean Gastric Cancer Assoc. 2008; 8:9–19.12. Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, et al. ACTS-GC Group. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007; 357:1810–1820.13. Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010; 11:439–449.14. Tsujimoto H, Ichikura T, Ono S, Sugasawa H, Hiraki S, Sakamoto N, et al. Impact of postoperative infection on long-term survival after potentially curative resection for gastric cancer. Ann Surg Oncol. 2009; 16:311–318.15. Pietrantonio F, De Braud F, Da Prat V, Perrone F, Pierotti MA, Gariboldi M, et al. A review on biomarkers for prediction of treatment outcome in gastric cancer. Anticancer Res. 2013; 33:1257–1266.16. Takeuchi H, Kakeji Y, Maehara Y. Time-dependent relevance of prognostic factors in patients with gastric cancer. Hepatogastroenterology. 2008; 55:779–781.17. Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 2nd English Edition. Gastric Cancer. 1998; 1:10–24.18. Sobin LH, Wittekind C, editors. TNM Classification of Malignant Tumors. 6th ed. New York: Wiley-Liss;2002.19. Hermans J, Bonenkamp JJ, Boon MC, Bunt AM, Ohyama S, Sasako M, et al. Adjuvant therapy after curative resection for gastric cancer: meta-analysis of randomized trials. J Clin Oncol. 1993; 11:1441–1447.20. Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996; 15:361–387.21. Miller R, Siegmund D. Maximally selected chi square statistics. Biometrics. 1982; 38:1011–1016.22. R Development CORE TEAM. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing;2010.23. Platt RW, Joseph KS, Ananth CV, Grondines J, Abrahamowicz M, Kramer MS. A proportional hazards model with timedependent covariates and time-varying effects for analysis of fetal and infant death. Am J Epidemiol. 2004; 160:199–206.24. Gilchrist KW, Gray R, Fowble B, Tormey DC, Taylor SG 4th. Tumor necrosis is a prognostic predictor for early recurrence and death in lymph node-positive breast cancer: a 10-year follow-up study of 728 Eastern Cooperative Oncology Group patients. J Clin Oncol. 1993; 11:1929–1935.25. Bolard P, Quantin C, Esteve J, Faivre J, Abrahamowicz M. Modelling time-dependent hazard ratios in relative survival: application to colon cancer. J Clin Epidemiol. 2001; 54:986–996.26. Berger U, Schäfer J, Ulm K. Dynamic Cox modelling based on fractional polynomials: time-variations in gastric cancer prognosis. Stat Med. 2003; 22:1163–1180.27. Ng'andu NH. An empirical comparison of statistical tests for assessing the proportional hazards assumption of Cox's model. Stat Med. 1997; 16:611–626.28. Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994; 81:515–526.29. Schoenfeld D. Chi-squared goodness-of-fit tests for the proportional hazards regression model. Biometrika. 1980; 67:145–153.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Tumor Size as a Prognostic Factor in Gastric Cancer Patient
- Prognostic Factors on Overall Survival in Lymph Node Negative Gastric Cancer Patients Who Underwent Curative Resection
- Prognostic Factors for Advanced Gastric Cancer: Stage- stratified Analysis of Patients who Underwent Curative Resection
- Utility of Surgical Resection in the Management of Metachronous Krukenberg's Tumors of Gastric Origin
- Neoadjuvant Chemotherapy in Asian Patients With Locally Advanced Gastric Cancer