Pediatr Infect Vaccine.
2017 Apr;24(1):16-22. 10.14776/piv.2017.24.1.16.
Differences in Clinical Manifestations and Treatment Responses in Influenza Type A and B in a Single Hospital during 2013 to 2015
- Affiliations
-
- 1Department of Pediatrics, Dongguk University Ilsan Hospital, Dongguk University College of Medicine, Goyang, the Republic of Korea. silbear@hanmail.net
- KMID: 2390877
- DOI: http://doi.org/10.14776/piv.2017.24.1.16
Abstract
- PURPOSE
We suspect there is a difference in the clinical manifestations and treatment response to antiviral drugs for influenza A and B. This study was conducted to investigate this difference.
METHODS
We collected information on pediatric patients, infected with the influenza virus, admitted to Dongguk University Ilsan Hospital from October 2013 to May 2015. We investigated the clinical manifestations of influenza and differences in treatment response to oseltamivir treatment for the two types of influenza.
RESULTS
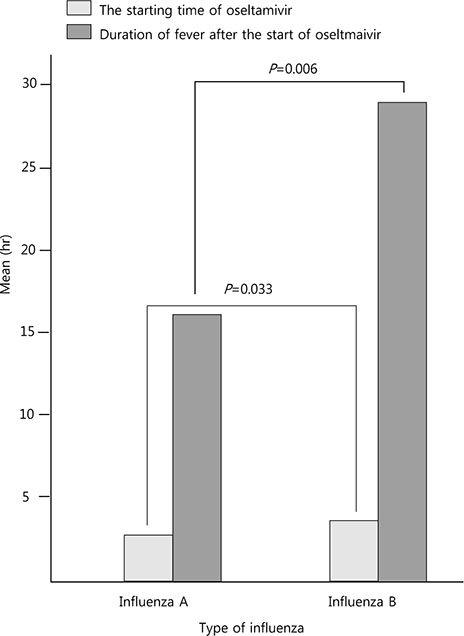
A total of 138 patients were included. The mean age was 3.5±4.0 years. When comparing the diseases associated with influenza A and B, croup (19.2% vs. 1.7%, P=0.001) was more common with influenza A infection. Myositis (0% vs. 6.7%, P=0.021) and gastroenteritis (29.5% vs. 46.7%, P=0.038) were more common with influenza B infection. When comparing the total fever duration from the start of oseltamivir administration, patients treated with oseltamivir within 2 days of fever had the shortest duration. Among the patients treated with oseltamivir, the duration of fever, after the start of oseltamivir treatment, for was shorter for influenza A infection than for influenza B infection (16.0±19.1 hours vs. 28.9±27.9 hours, P=0.006).
CONCLUSIONS
There appear to be differences in the accompanying diseases and antiviral medication responses between the two types of influenza. It is important to administer oseltamivir within 2 days of fever.
Keyword
MeSH Terms
Figure
Reference
-
1. Kim MS, Sung HW, Bae EY, Han SB, Jeong DC, Kang JH. The clinical characteristics of influenza B infection during the 2011–2012 influenza season. Korean J Pediatr Infect Dis. 2013; 20:89–97.
Article2. Choi JW, Cho HJ, Kim HM, Hahn S. Clinical and laboratory findings of the 2012 winter seasonal influenza A and B outbreak at a single institution. Korean J Pediatr Infect Dis. 2014; 21:1–8.
Article3. Sugaya N, Mitamura K, Yamazaki M, Tamura D, Ichikawa M, Kimura K, et al. Lower clinical effectiveness of oseltamivir against influenza B contrasted with influenza A infection in children. Clin Infect Dis. 2007; 44:197–202.
Article4. Kang TG, Kim MJ, Kim BG, An HS, Yun HJ, Choi EJ, et al. Comparisons of clinical features among influenza A (H1N1) and seasonal influenza A and B during 2009 to 2010 at a single institution. Pediatr Allergy Respir Dis. 2011; 21:269–276.
Article5. Daley AJ, Nallusamy R, Isaacs D. Comparison of influenza A and influenza B virus infection in hospitalized children. J Paediatr Child Health. 2000; 36:332–335.
Article6. Noma S. Acute myositis associated with influenza. Nihon Rinsho. 2006; 64:1921–1923.7. Youn SE, Chun JH, Lee KS, Rha YH, Choi SH. Clinical characteristics of influenza B virus in children and the efficacy of oseltamivir: data from two university hospitals. Korean J Pediatr Infect Dis. 2014; 21:199–206.
Article8. Nicholson KG, Aoki FY, Osterhaus AD, Trottier S, Carewicz O, Mercier CH, et al. Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Neuraminidase Inhibitor Flu Treatment Investigator Group. Lancet. 2000; 355:1845–1850.
Article9. Mitamura K, Sugaya N, Nirasawa M, Shinjoh M, Takeuchi Y. Effectiveness of oseltamivir treatment against influenza type A and type B infection in children. Kansenshogaku Zasshi. 2002; 76:946–952.
Article10. Sheu TG, Deyde VM, Okomo-Adhiambo M, Garten RJ, Xu X, Bright RA, et al. Surveillance for neuraminidase inhibitor resistance among human influenza A and B viruses circulating worldwide from 2004 to 2008. Antimicrob Agents Chemother. 2008; 52:3284–3292.
Article11. Burnham AJ, Baranovich T, Govorkova EA. Neuraminidase inhibitors for influenza B virus infection: efficacy and resistance. Antiviral Res. 2013; 100:520–534.
Article12. Fujisaki S, Takashita E, Yokoyama M, Taniwaki T, Xu H, Kishida N, et al. A single E105K mutation far from the active site of influenza B virus neuraminidase contributes to reduced susceptibility to multiple neuraminidase-inhibitor drugs. Biochem Biophys Res Commun. 2012; 429:51–56.
Article13. Ministry of Food and Drug Safety. Proven domestic influenza vaccine safety, efficacy and effectiveness [Internet]. Cheongju: Ministry of Food and Drug Safety;2015. cited 2016 Dec 22. Available from: http://www.mfds.go.kr/index.do?mid=675&seq=26946&cmd=v.14. Ki HO, Kim IH, Cho EH, Kang MG, Chu H. Korean influenza sentinel surveillance report, 2013–2014. Public Health Wkly Rep. 2014; 7:1089–1098.15. Ki HO, Kim IH, Cho EH, Kang MG, Chu H, Lee JY. Korean influenza sentinel surveillance report, 2014–2015. Public Health Wkly Rep. 2015; 8:1096–1106.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prevention and Treatment of Influenza
- Influenza Vaccine
- Host Immune Responses Against Type A Influenza Viruses
- Epidemiology, clinical manifestations, and management of pandemic novel Influenza A (H1N1)
- Survey on the Effects of Educational Intervention in Parents' Perceptions and Decisions Regarding Influenza Vaccination for Their Children Aged 6–59 Months