Pediatr Gastroenterol Hepatol Nutr.
2017 Sep;20(3):178-185. 10.5223/pghn.2017.20.3.178.
Cytomegalovirus Infection under a Hybrid Strategy in Pediatric Liver Transplantation: A Single-Center Experience
- Affiliations
-
- 1Department of Pediatric, Asan Medical Center Children's Hospital, University of Ulsan College of Medicine, Seoul, Korea. seakhee.oh@amc.seoul.kr
- 2Department of Pediatric Surgery, Asan Medical Center Children's Hospital, University of Ulsan College of Medicine, Seoul, Korea.
- KMID: 2390549
- DOI: http://doi.org/10.5223/pghn.2017.20.3.178
Abstract
- PURPOSE
To evaluate the outcomes of a hybrid prophylactic strategy to prevent cytomegalovirus (CMV) disease in pediatric liver transplantation (LT) patients.
METHODS
CMV DNAemia was regularly monitored by quantitative nucleic acid amplification test (QNAT) and was quantified in all children. CMV infection and disease were defined according to the International Consensus Guidelines. The hybrid strategy against CMV infection consisted of universal 3-week prophylaxis and preemptive treatment of intravenous ganciclovir regardless of the recipient's serostatus.
RESULTS
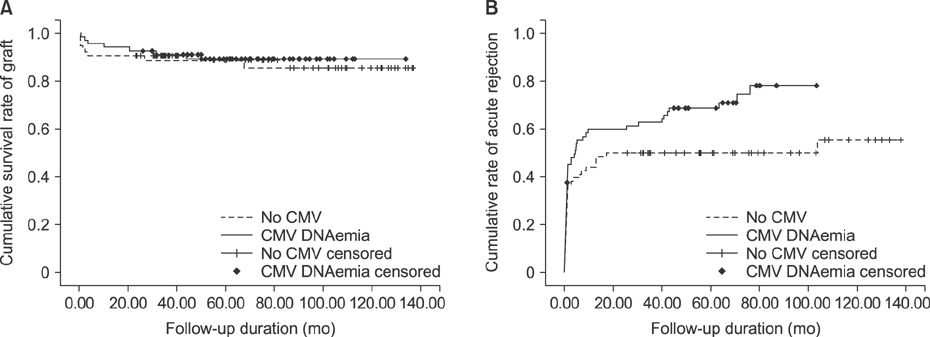
A total of 143 children who underwent living donor LT were managed using the hybrid strategy. The overall incidence of CMV infection by QNAT was 48.3% (n=69/143). The highest CMV DNAemia positivity was observed in 49.2% (n=60/122) of children in the D+/R+ group, followed by 46.7% (n=7/15) in the D+/R− group. CMV disease was noted in 26.1% (n=18/69) patients. Forty-three (62.3%) children had undergone preemptive therapy consisting of intravenous ganciclovir. No symptomatic patients developed tissue-invasive disease, resulting in no CMV-associated mortality.
CONCLUSION
The incidence of CMV infection was high in pediatric LT patients despite the hybrid strategy. However, tissue-invasive disease in pediatric LT did not occur.
Keyword
MeSH Terms
Figure
Reference
-
1. Humar A, Snydman D;. Cytomegalovirus in solid organ transplant recipients. Am J Transplant. 2009; 9:Suppl 4. S78–S86.
Article2. Fishman JA. Infection in solid-organ transplant recipients. N Engl J Med. 2007; 357:2601–2614.
Article3. Kim JM, Kim SJ, Joh JW, Kwon CH, Song S, Shin M, et al. Is cytomegalovirus infection dangerous in cytomegalovirus-seropositive recipients after liver transplantation? Liver Transpl. 2011; 17:446–455.
Article4. Madan RP, Campbell AL, Shust GF, Kahn AR, Wistinghausen B, Posada R, et al. A hybrid strategy for the prevention of cytomegalovirus-related complications in pediatric liver transplantation recipients. Transplantation. 2009; 87:1318–1324.
Article5. George MJ, Snydman DR, Werner BG, Griffith J, Falagas ME, Dougherty NN, et al. The independent role of cytomegalovirus as a risk factor for invasive fungal disease in orthotopic liver transplant recipients. Boston Center for Liver Transplantation CMVIG-Study Group. Cytogam, MedImmune, Inc. Gaithersburg, Maryland. Am J Med. 1997; 103:106–113.
Article6. Mañez R, Breinig MC, Linden P, Wilson J, Torre-Cisneros J, Kusne S, et al. Posttransplant lymphoproliferative disease in primary Epstein-Barr virus infection after liver transplantation: the role of cytomegalovirus disease. J Infect Dis. 1997; 176:1462–1467.
Article7. Gupta P, Hart J, Cronin D, Kelly S, Millis JM, Brady L. Risk factors for chronic rejection after pediatric liver transplantation. Transplantation. 2001; 72:1098–1102.
Article8. Kotton CN, Kumar D, Caliendo AM, Asberg A, Chou S, Danziger-Isakov L, et al. Updated international consensus guidelines on the management of cytomegalovirus in solid-organ transplantation. Transplantation. 2013; 96:333–360.
Article9. Razonable RR, Hayden RT. Clinical utility of viral load in management of cytomegalovirus infection after solid organ transplantation. Clin Microbiol Rev. 2013; 26:703–727.
Article10. Lee SO, Razonable RR. Current concepts on cytomegalovirus infection after liver transplantation. World J Hepatol. 2010; 2:325–336.
Article11. Bedel AN, Hemmelgarn TS, Kohli R. Retrospective review of the incidence of cytomegalovirus infection and disease after liver transplantation in pediatric patients: comparison of prophylactic oral ganciclovir and oral valganciclovir. Liver Transpl. 2012; 18:347–354.
Article12. Saitoh A, Sakamoto S, Fukuda A, Shigeta T, Kakiuchi T, Kamiyama S, et al. A universal preemptive therapy for cytomegalovirus infections in children after live-donor liver transplantation. Transplantation. 2011; 92:930–935.
Article13. Lapidus-Krol E, Shapiro R, Amir J, Davidovits M, Steinberg R, Mor E, et al. The efficacy and safety of valganciclovir vs. oral ganciclovir in the prevention of symptomatic CMV infection in children after solid organ transplantation. Pediatr Transplant. 2010; 14:753–760.
Article14. Krampe K, Briem-Richter A, Fischer L, Nashan B, Ganschow R. The value of immunoprophylaxis for cytomegalovirus infection with intravenous immunoglobulin in pediatric liver transplant recipients receiving a low-dose immunosupressive regimen. Pediatr Transplant. 2010; 14:67–71.
Article15. Oh SH, Kim KM, Kim DY, Lee YJ, Rhee KW, Jang JY, et al. Long-term outcomes of pediatric living donor liver transplantation at a single institution. Pediatr Transplant. 2010; 14:870–878.
Article16. Mendez J, Espy M, Smith TF, Wilson J, Wiesner R, Paya CV. Clinical significance of viral load in the diagnosis of cytomegalovirus disease after liver transplantation. Transplantation. 1998; 65:1477–1481.
Article17. Li H, Dummer JS, Estes WR, Meng S, Wright PF, Tang YW. Measurement of human cytomegalovirus loads by quantitative real-time PCR for monitoring clinical intervention in transplant recipients. J Clin Microbiol. 2003; 41:187–191.
Article18. Demetris A, Adams D, Bellamy C, Blakolmer K, Clouston A, Dhillon AP, et al. Update of the international banff schema for liver allograft rejection: working recommendations for the histopathologic staging and reporting of chronic rejection. An international panel. Hepatology. 2000; 31:792–799.
Article19. Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988; 16:128–140.
Article20. Bowman JS, Green M, Scantlebury VP, Todo S, Tzakis A, Iwatsuki S, et al. OKT3 and viral disease in pediatric liver transplant recipients. Clin Transplant. 1991; 5:294–300.21. Danziger-Isakov L, Bucavalas J. Current prevention strategies against cytomegalovirus in the studies in pediatric liver transplantation (SPLIT) centers. Am J Transplant. 2014; 14:1908–1911.
Article22. Smith JM, Corey L, Bittner R, Finn LS, Healey PJ, Davis CL, et al. Subclinical viremia increases risk for chronic allograft injury in pediatric renal transplantation. J Am Soc Nephrol. 2010; 21:1579–1586.
Article23. Razonable RR, Humar A;. Cytomegalovirus in solid organ transplantation. Am J Transplant. 2013; 13:Suppl 4. 93–106.
Article24. Le Page AK, Jager MM, Kotton CN, Simoons-Smit A, Rawlinson WD. International survey of cytomegalovirus management in solid organ transplantation after the publication of consensus guidelines. Transplantation. 2013; 95:1455–1460.
Article25. Paya C, Humar A, Dominguez E, Washburn K, Blumberg E, Alexander B, et al. Efficacy and safety of valganciclovir vs. oral ganciclovir for prevention of cytomegalovirus disease in solid organ transplant recipients. Am J Transplant. 2004; 4:611–620.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- ABO incompatibility is a risk factor for cytomegalovirus infection with living donor liver transplantation
- Current Status of Pediatric Liver Transplantation
- Clinical impact and risk factors for cytomegalovirus infection in deceased donor liver transplantation without prophylaxis: Single center experience
- Indication of Pediatric Liver Transplantation
- Pediatric Liver Transplantation