Anat Cell Biol.
2017 Mar;50(1):17-25. 10.5115/acb.2017.50.1.17.
Morphology of cat vomeronasal organ non-sensory epithelium during postnatal development
- Affiliations
-
- 1Department of Histology, Faculty of Medicine, Assiut University, Assiut, Egypt. selgar1@hotmail.com
- KMID: 2390422
- DOI: http://doi.org/10.5115/acb.2017.50.1.17
Abstract
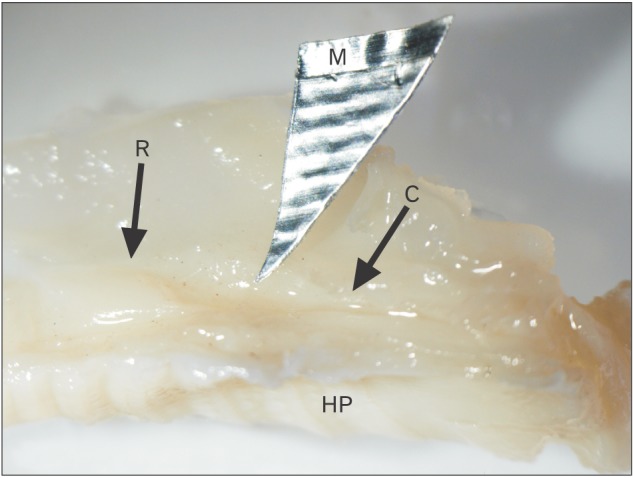
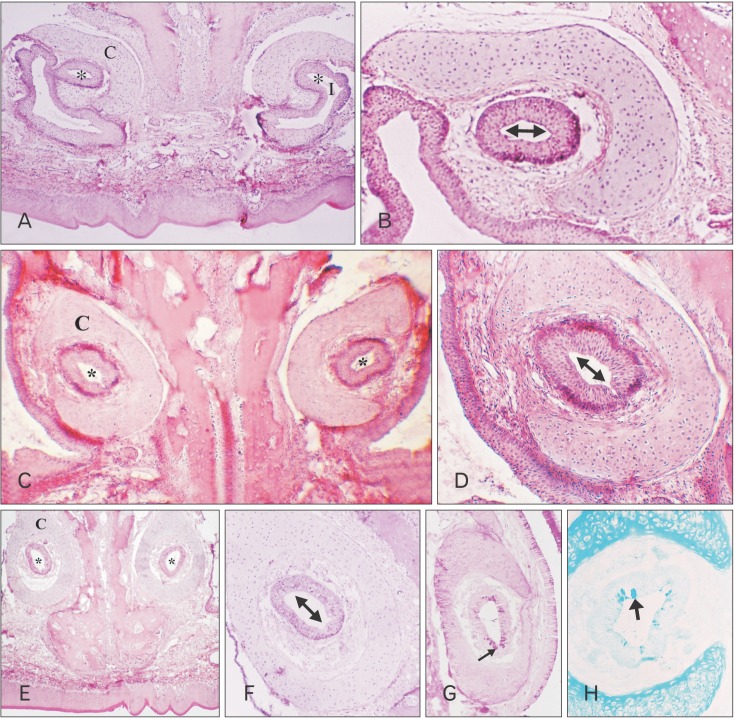
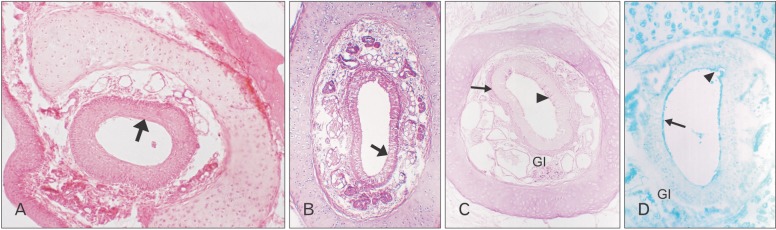
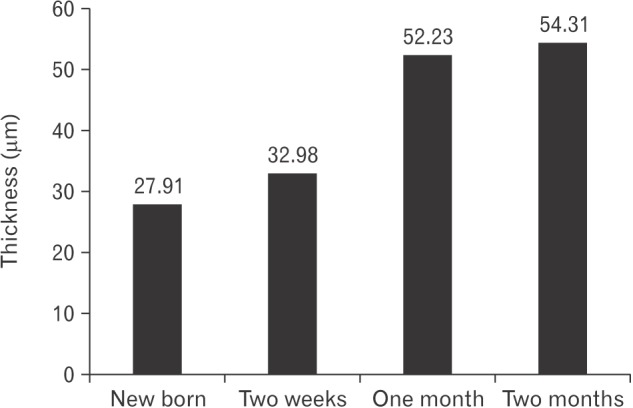
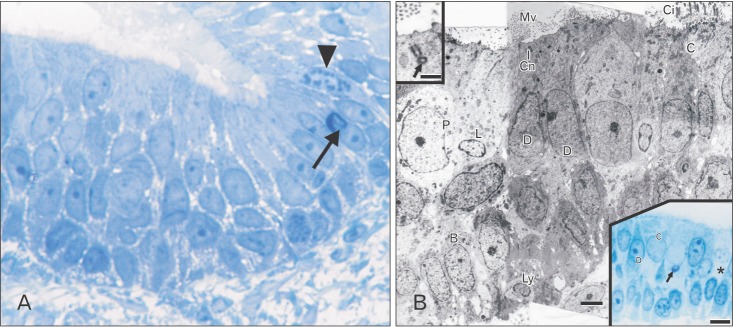
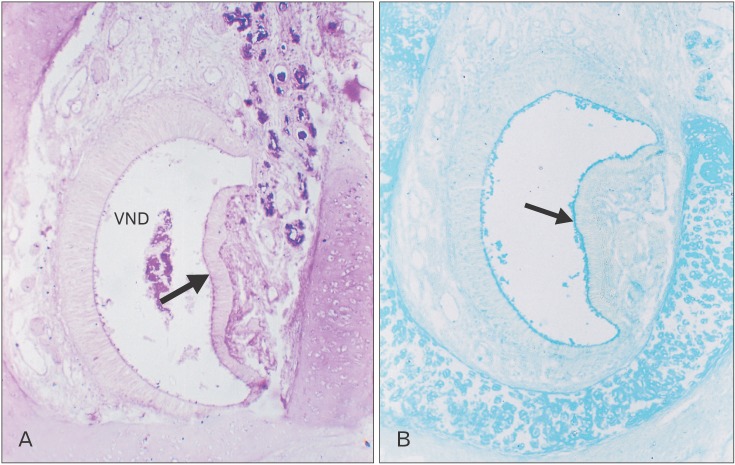
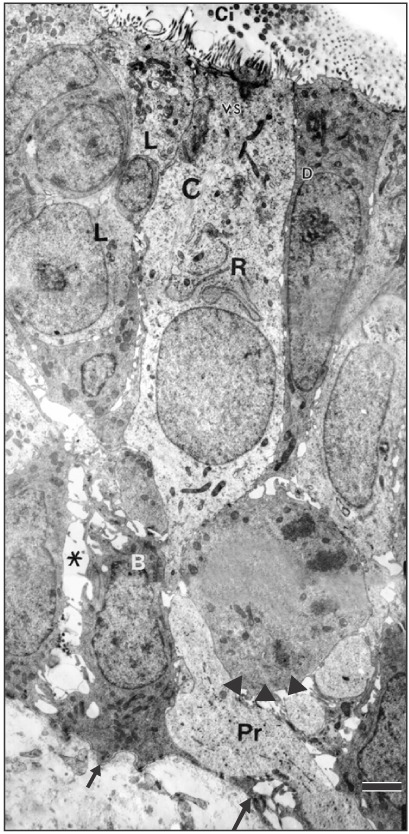
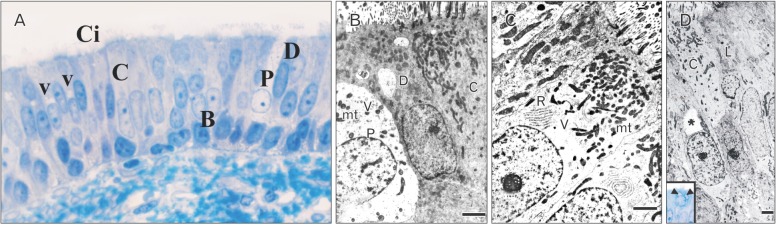
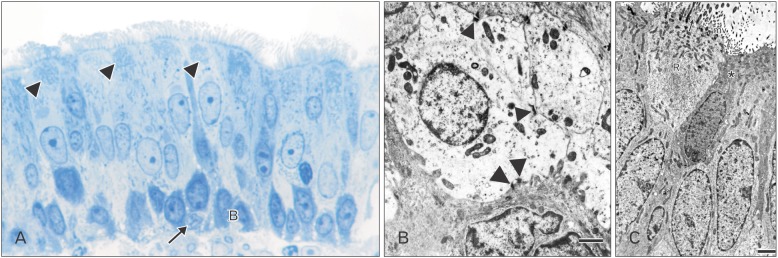
- The vomeronasal organ has an important role in mammal's social and sexual behaviours. In addition, it mediates defensive behavior through detection of protein pheromone homologues. In this work, a detailed morphological description of the postnatal development of the non-sensory epithelium (NSE) lining the vomeronasal duct (VND) of the female cat is provided using various histological techniques. The study focused on newborn, 2 weeks, 4 weeks, and 8 weeks of postnatal ages using four animals for each age. We report here for the first time that three types of NSE line the rostral segment of the VND; nonkeratinized stratified squamous epithelium, stratified cuboidal epithelium, and ciliated pseudo stratified columnar ciliated epithelium with goblet cells and that the VND undergoes 90° a change in its its axis from the vertical position caudally to the horizontal position rostral. The NSE which lines the lateral side of the VND middle segment is consists of cliated pseudostratified columnar epithelium without goblet cells. In addition to basal cells, the NSE contains ciliated and three types of nonciliated columnar epithelial cells (dark, light, and unstained). Mitotic figures were observed only in the basal cells layer during the first 2 weeks of postnatal development. Intraepithelial invading inflammatory cells were uncommon. Scanning electron microscopy revealed unevenly distributed long cilia intermingled with microvillar processes and intervening short microvillar processes. These projecting cilia and microvilli revealed a gradual increase in their height during development toward maturity.
Keyword
MeSH Terms
Figure
Reference
-
1. Brennan PA. The vomeronasal system. Cell Mol Life Sci. 2001; 58:546–555. PMID: 11361090.2. Keverne EB. Pheromones, vomeronasal function, and gender-specific behavior. Cell. 2002; 108:735–738. PMID: 11955427.3. Meredith M. Chronic recording of vomeronasal pump activation in awake behaving hamsters. Physiol Behav. 1994; 56:345–354. PMID: 7938248.4. Garrosa M, Coca S, Mora OA. Histological development of the vomeronasal complex in the pre- and postnatal rat. Acta Otolaryngol. 1986; 102:291–301. PMID: 3776523.5. Garrosa M, Coca S. Postnatal development of the vomeronasal epithelium in the rat: an ultrastructural study. J Morphol. 1991; 208:257–269. PMID: 1920442.6. Garrosa M, Iñiguez C, Fernandez JM, Gayoso MJ. Developmental stages of the vomeronasal organ in the rat: a light and electron microscopic study. J Hirnforsch. 1992; 33:123–132. PMID: 1447518.7. Weiler E. Postnatal development of the rat vomeronasal organ. Chem Senses. 2005; 30(Suppl 1):i127–i128. PMID: 15738073.8. Nakano T, Hasegawa K, Tomatsu M, Muto H. Postnatal transformation and location of mitoses in the epithelium lining the mouse vomeronasal organ. Okajimas Folia Anat Jpn. 1990; 67:81–88. PMID: 2216316.9. Elgayar SA, Eltony SA, Othman MA. Morphology of non-sensory epithelium during post-natal development of the rabbit vomeronasal organ. Anat Histol Embryol. 2014; 43:282–293. PMID: 23931650.10. Taniguchi K, Taniguchi K, Mochizuki K. Developmental studies on the vomeronasal organ in the golden hamster. Nihon Juigaku Zasshi. 1982; 44:709–716. PMID: 7161988.11. Taniguchi K, Taniguchi K. Embryonic and postnatal differentiation of olfactory epithelium and vomeronasal organ in the Syrian hamster. J Vet Med Sci. 2008; 70:57–64. PMID: 18250573.12. Irwin PJ. Companion animal parasitology: a clinical perspective. Int J Parasitol. 2002; 32:581–593. PMID: 11943231.13. Salazar I, Sanchez Quinteiro P, Cifuentes JM, Garcia Caballero T. The vomeronasal organ of the cat. J Anat. 1996; 188(Pt 2):445–454. PMID: 8621344.14. Salazar I, Sánchez-Quinteiro P. A detailed morphological study of the vomeronasal organ and the accessory olfactory bulb of cats. Microsc Res Tech. 2011; 74:1109–1120. PMID: 21484946.15. Drury RA, Wallington EA. Carelton's histological technique. 5th ed. Oxford: Oxford University Press;1980. p. 237–239.16. Gupta PD. Ultrastructural study on semithin section. Sci Tools. 1983; 30:6–7.17. Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963; 17:208–212. PMID: 13986422.18. Naguro T, Breipohl W. The vomeronasal epithelia of NMRI mouse: a scanning electron-microscopic study. Cell Tissue Res. 1982; 227:519–534. PMID: 7151135.19. Meisami E, Louie J, Hudson R, Distel H. A morphometric comparison of the olfactory epithelium of newborn and weanling rabbits. Cell Tissue Res. 1990; 262:89–97. PMID: 2257619.20. Coppola DM, Budde J, Millar L. The vomeronasal duct has a protracted postnatal development in the mouse. J Morphol. 1993; 218:59–64. PMID: 8230236.21. Coppola DM, Millar LC. Stimulus access to the accessory olfactory system in the prenatal and perinatal rat. Neuroscience. 1994; 60:463–468. PMID: 8072692.22. Steinberg H. Description de l'organe de Jacobson chez un foetus de chat. Anat Anz. 1912; 42:466–472.23. Kratzing JE. The olfactory apparatus of the bandicoot (Isoodon macrourus): fine structure and presence of a septal olfactory organ. J Anat. 1978; 125:601–613. PMID: 640961.24. Adams DR, Wiekamp MD. The canine vomeronasal organ. J Anat. 1984; 138(Pt 4):771–787. PMID: 6746408.25. Ponzio R. Sistema de endomembranas. In : De Robertis ED, Hib J, Ponzio R, editors. Biologia Cellular y Molecular. Buenos Aires: El Ateneo;1996. p. 221–273.26. Ferrari CC, Aldana Marcos HJ, Carmanchahi PD, Affanni JM. Olfactory mucosa of the South American armadillo Chaetophractus villosus: an ultrastructural study. Anat Rec. 1998; 252:325–339. PMID: 9811211.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Histochemical study of lectin-binding patterns in the rat vomeronasal organ during postnatal development
- A morphological study of vomeronasal organ of Korean black goat (Capra aegagrus hircus)
- Histochemical Localization of NADPH-Diaphorase in the Rat Vomeronasal Organ
- Histochemical Characterization of the Lectin-binding Sites in the Equine Vomeronasal Organ
- Sensory Stimulation-dependent Npas4 Expression in the Olfactory Bulb during Early Postnatal Development