J Korean Ophthalmol Soc.
2017 Sep;58(9):1017-1022. 10.3341/jkos.2017.58.9.1017.
Analysis of Microorganisms and Antibiotics Susceptibility in Dacryocystitis
- Affiliations
-
- 1Department of Ophthalmology, Chonbuk National University Medical School, Jeonju, Korea. ahnmin@jbnu.ac.kr
- 2Research Institute of Clinical Medicine, Chonbuk National University, Jeonju, Korea.
- 3Biomedical Research Institute, Chonbuk National University Hospital, Jeonju, Korea.
- KMID: 2390063
- DOI: http://doi.org/10.3341/jkos.2017.58.9.1017
Abstract
- PURPOSE
This article analyzes the microorganisms and antibiotics susceptibility in dacryocystitis.
METHODS
In this study, patients who were diagnosed with acute and chronic dacryocystitis with nasolacrimal duct obstruction were selected and underwent endoscopic endonasal dacryocystorhinostomy. Cultures were obtained from the lacrimal sac during operation from January 2008 to January 2016, and were used to analyze the microorganisms and antibiotics susceptibility.
RESULTS
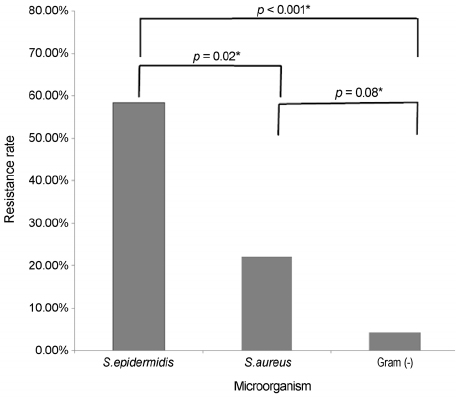
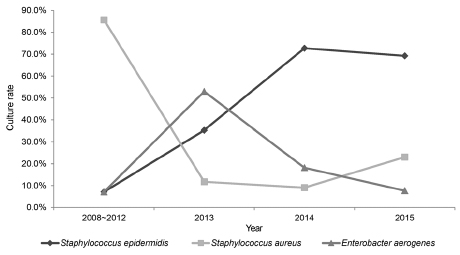
The 67 patients, 9 were diagnosed with acute dacryocystitis and 58 were diagnosed with chronic dacryocystitis. Among them, 64 cases showed bacterial growth (95.5%). The most frequently detected bacteria was Staphylococcus epidermidis (S. epidermidis) (33.8%), followed by Staphylococcus aureus (S. aureus) (25.4%) and Enterobacter aerogenes (18.3%). S. epidermidis had the most powerful resistance to ciprofloxacin compared to the other bacteria (58.3%, p = 0.02). Except for S. epidermidis and S. aureus, the other bacteria responded to ciprofloxacin and gentamycin.
CONCLUSIONS
As a causative microorganism of dacryocystitis, S. epidermidis is becoming more prominent, and it is thought that S. epidermidis may be resistant to quinolones (i.e., broad-spectrum antibiotics). This resistance might be increasing the percentage of present S. epidermidis when viewed as a causal pathogen in dacryocystitis.
MeSH Terms
Figure
Reference
-
1. Pepose JS, Holland GN, Wilhelmus KR. Ocular Infection and Immunity. 1st ed. St. Louis: Mosby;1996. Vol. 1:p. 1346–1355.2. Hurwitz JJ, Rodgers KJ. Management of acquired dacryocystitis. Can J Ophthalmol. 1983; 18:213–216.3. Bartley GB. Acquired lacrimal drainage obstruction: an etiologic classification system, case reports, and a review of the literature. Part 1. Ophthal Plast Reconstr Surg. 1992; 8:237–242.4. Cahill KV, Burns JA. Management of acute dacryocystitis in adults. Ophthal Plast Reconstr Surg. 1993; 9:38–41. discussion 42.5. Hartikainen J, Lehtonen OP, Saari KM. Bacteriology of lacrimal duct obstruction in adults. Br J Ophthalmol. 1997; 81:37–40.6. Huber-Spitzy V, Steinkogler FJ, Huber E, et al. Acquired dacryocystitis: microbiology and conservative therapy. Acta Ophthalmol (Copenh). 1992; 70:745–749.7. Blicker JA, Buffam FV. Lacrimal sac, conjunctival, and nasal culture results in dacryocystorhinostomy patients. Ophthal Plast Reconstr Surg. 1993; 9:43–46.8. Coden DJ, Hornblass A, Haas BD. Clinical bacteriology of dacryocystitis in adults. Ophthal Plast Reconstr Surg. 1993; 9:125–131.9. Owji N, Khalili MR. Normalization of conjunctival flora after dacryocystorhinostomy. Ophthal Plast Reconstr Surg. 2009; 25:136–138.10. Can I, Aribal E, Yarangümeli A, et al. Changes in the conjunctival flora after conjunctivodacryocystorhinostomy (CDCR): a preliminary report. Eur J Ophthalmol. 1998; 8:142–147.11. Jung SK, Cho WK, Paik JS, Yang SW. The correlation between organisms cultured from the lacrimal sac and lacrimal punctum in dacryocystitis. J Korean Ophthalmol Soc. 2011; 52:897–901.12. Kim SE, Lee SJ, Lee SY, Yoon JS. Clinical significance of microbial growth on the surfaces of silicone tubes removed from dacryocystorhinostomy patients. Am J Ophthalmol. 2012; 153:253–257.e1.13. Park JY, Lee JS. Results of the cultured nasolacrimal polyurethane stents (Song's stent(R)) in nasolacrimal duct obstruction treatment. J Korean Ophthalmol Soc. 2015; 56:823–829.14. Patel JB, Cockerill FR, Alder J, et al. Performance standards for antimicrobial susceptibility testing; twenty-fourth informational supplement. 24th ed. Philadelphia: Clinical and Laboratory Standards Institute;2014. p. 38–39.15. Gaynor BD, Chidambaram JD, Cevallos V, et al. Topical ocular antibiotics induce bacterial resistance at extraocular sites. Br J Ophthalmol. 2005; 89:1097–1099.16. Bharathi MJ, Ramakrishnan R, Maneksha V, et al. Comparative bacteriology of acute and chronic dacryocystitis. Eye (Lond). 2008; 22:953–960.17. Assefa Y, Moges F, Endris M, et al. Bacteriological profile and drug susceptibility patterns in dacryocystitis patients attending Gondar University Teaching Hospital, Northwest Ethiopia. BMC Ophthalmol. 2015; 15:34.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Correlation between Organisms Cultured from the Lacrimal Sac and Lacrimal Punctum in Dacryocystitis
- A Case of Chronic Dacryocystitis Caused by Achromobacter Xylosoxidans
- Characteristics in Patients with and without Previous Dacryocystitis and Satisfaction after Endonasal Dacryocystorhinostomy
- Endoscopic Dacryocystorhinostomy for the Treatment of Lacrimal Sac Abscess
- Storage of the split-thickness skin piece using proper antibiotics