Korean J Physiol Pharmacol.
2017 Sep;21(5):487-493. 10.4196/kjpp.2017.21.5.487.
Inhibition of anterior cingulate cortex excitatory neuronal activity induces conditioned place preference in a mouse model of chronic inflammatory pain
- Affiliations
-
- 1Department of Biological Sciences, College of Natural Sciences, Seoul National University, Seoul 08826, Korea. kaang@snu.ac.kr
- 2Interdisciplinary Program in Neuroscience, College of Natural Sciences, Seoul National University, Seoul 08826, Korea.
- 3Department of Anatomy, Brain Science & Engineering Institute, Kyungpook National University School of Medicine, Daegu 41944, Korea.
- 4Center for Neuron and Disease, Frontier Institutes of Life Science and of Science and Technology, Xi'an Jiaotong University, Xi'an 710049, China.
- 5Department of Physiology, Faculty of Medicine, University of Toronto, 1 King's College Circle, Toronto, Ontario M5S 1A8, Canada.
- KMID: 2388694
- DOI: http://doi.org/10.4196/kjpp.2017.21.5.487
Abstract
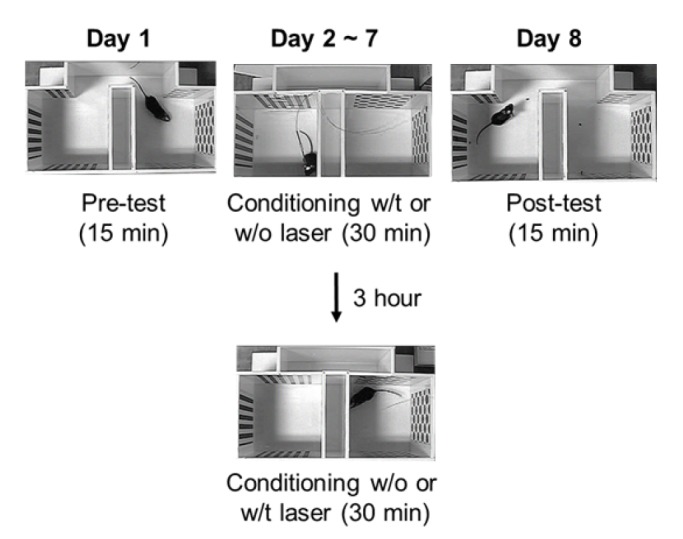
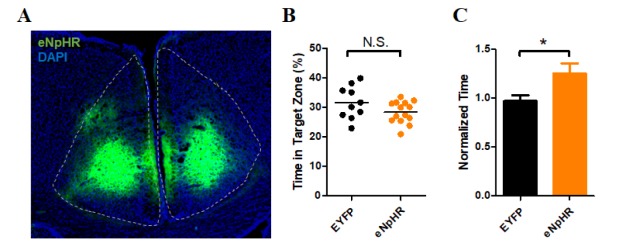
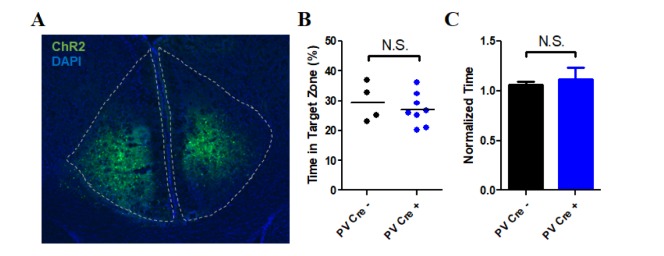
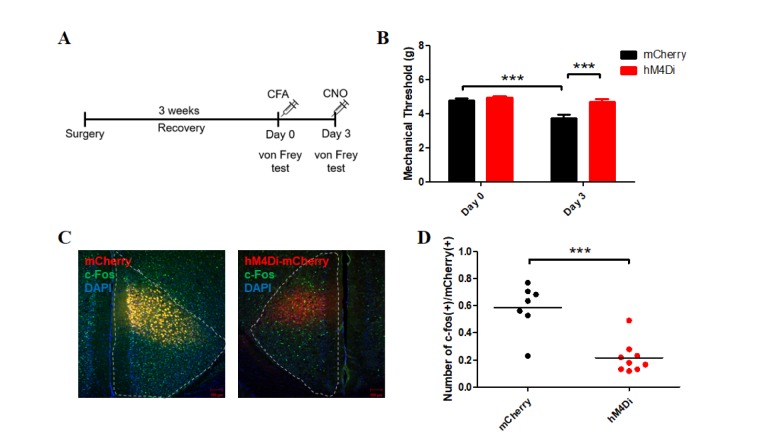
- The anterior cingulate cortex (ACC) is known for its role in perception of nociceptive signals and the associated emotional responses. Recent optogenetic studies, involving modulation of neuronal activity in the ACC, show that the ACC can modulate mechanical hyperalgesia. In the present study, we used optogenetic techniques to selectively modulate excitatory pyramidal neurons and inhibitory interneurons in the ACC in a model of chronic inflammatory pain to assess their motivational effect in the conditioned place preference (CPP) test. Selective inhibition of pyramidal neurons induced preference during the CPP test, while activation of parvalbumin (PV)-specific neurons did not. Moreover, chemogenetic inhibition of the excitatory pyramidal neurons alleviated mechanical hyperalgesia, consistent with our previous result. Our results provide evidence for the analgesic effect of inhibition of ACC excitatory pyramidal neurons and a prospective treatment for chronic pain.
MeSH Terms
Figure
Cited by 1 articles
-
Imaging and analysis of genetically encoded calcium indicators linking neural circuits and behaviors
Jihae Oh, Chiwoo Lee, Bong-Kiun Kaang
Korean J Physiol Pharmacol. 2019;23(4):237-249. doi: 10.4196/kjpp.2019.23.4.237.
Reference
-
1. Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000; 4:215–222. PMID: 10827444.
Article2. Allman JM, Hakeem A, Erwin JM, Nimchinsky E, Hof P. The anterior cingulate cortex. The evolution of an interface between emotion and cognition. Ann N Y Acad Sci. 2001; 935:107–117. PMID: 11411161.3. Shenhav A, Botvinick MM, Cohen JD. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron. 2013; 79:217–240. PMID: 23889930.
Article4. Einarsson EÖ, Pors J, Nader K. Systems reconsolidation reveals a selective role for the anterior cingulate cortex in generalized contextual fear memory expression. Neuropsychopharmacology. 2015; 40:480–487. PMID: 25091528.
Article5. Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004; 304:881–883. PMID: 15131309.
Article6. Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci. 2011; 15:85–93. PMID: 21167765.
Article7. Bliss TV, Collingridge GL, Kaang BK, Zhuo M. Synaptic plasticity in the anterior cingulate cortex in acute and chronic pain. Nat Rev Neurosci. 2016; 17:485–496. PMID: 27307118.
Article8. Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nat Rev Neurosci. 2005; 6:533–544. PMID: 15995724.
Article9. Fuchs PN, Peng YB, Boyette-Davis JA, Uhelski ML. The anterior cingulate cortex and pain processing. Front Integr Neurosci. 2014; 8:35. PMID: 24829554.
Article10. Dunckley P, Wise RG, Aziz Q, Painter D, Brooks J, Tracey I, Chang L. Cortical processing of visceral and somatic stimulation: differentiating pain intensity from unpleasantness. Neuroscience. 2005; 133:533–542. PMID: 15896917.
Article11. Johansen JP, Fields HL. Glutamatergic activation of anterior cingulate cortex produces an aversive teaching signal. Nat Neurosci. 2004; 7:398–403. PMID: 15004562.
Article12. Peyron R, Laurent B, García-Larrea L. Functional imaging of brain responses to pain. A review and meta-analysis (2000). Neurophysiol Clin. 2000; 30:263–288. PMID: 11126640.
Article13. Strigo IA, Duncan GH, Boivin M, Bushnell MC. Differentiation of visceral and cutaneous pain in the human brain. J Neurophysiol. 2003; 89:3294–3303. PMID: 12611986.
Article14. Talbot JD, Marrett S, Evans AC, Meyer E, Bushnell MC, Duncan GH. Multiple representations of pain in human cerebral cortex. Science. 1991; 251:1355–1358. PMID: 2003220.
Article15. Kang SJ, Liu MG, Chen T, Ko HG, Baek GC, Lee HR, Lee K, Collingridge GL, Kaang BK, Zhuo M. Plasticity of metabotropic glutamate receptor-dependent long-term depression in the anterior cingulate cortex after amputation. J Neurosci. 2012; 32:11318–11329. PMID: 22895715.
Article16. Li XY, Ko HG, Chen T, Descalzi G, Koga K, Wang H, Kim SS, Shang Y, Kwak C, Park SW, Shim J, Lee K, Collingridge GL, Kaang BK, Zhuo M. Alleviating neuropathic pain hypersensitivity by inhibiting PKMzeta in the anterior cingulate cortex. Science. 2010; 330:1400–1404. PMID: 21127255.17. Shyu BC, Vogt BA. Short-term synaptic plasticity in the nociceptive thalamic-anterior cingulate pathway. Mol Pain. 2009; 5:51. PMID: 19732417.
Article18. Wei F, Li P, Zhuo M. Loss of synaptic depression in mammalian anterior cingulate cortex after amputation. J Neurosci. 1999; 19:9346–9354. PMID: 10531439.
Article19. Wu LJ, Toyoda H, Zhao MG, Lee YS, Tang J, Ko SW, Jia YH, Shum FW, Zerbinatti CV, Bu G, Wei F, Xu TL, Muglia LJ, Chen ZF, Auberson YP, Kaang BK, Zhuo M. Upregulation of forebrain NMDA NR2B receptors contributes to behavioral sensitization after inflammation. J Neurosci. 2005; 25:11107–11116. PMID: 16319310.
Article20. Tye KM, Deisseroth K. Optogenetic investigation of neural circuits underlying brain disease in animal models. Nat Rev Neurosci. 2012; 13:251–266. PMID: 22430017.
Article21. Yizhar O, Fenno LE, Davidson TJ, Mogri M, Deisseroth K. Optogenetics in neural systems. Neuron. 2011; 71:9–34. PMID: 21745635.
Article22. Iyer SM, Montgomery KL, Towne C, Lee SY, Ramakrishnan C, Deisseroth K, Delp SL. Virally mediated optogenetic excitation and inhibition of pain in freely moving nontransgenic mice. Nat Biotechnol. 2014; 32:274–278. PMID: 24531797.
Article23. Hickey L, Li Y, Fyson SJ, Watson TC, Perrins R, Hewinson J, Teschemacher AG, Furue H, Lumb BM, Pickering AE. Optoactivation of locus ceruleus neurons evokes bidirectional changes in thermal nociception in rats. J Neurosci. 2014; 34:4148–4160. PMID: 24647936.
Article24. Lee M, Manders TR, Eberle SE, Su C, D’amour J, Yang R, Lin HY, Deisseroth K, Froemke RC, Wang J. Activation of corticostriatal circuitry relieves chronic neuropathic pain. J Neurosci. 2015; 35:5247–5259. PMID: 25834050.
Article25. Wang GQ, Cen C, Li C, Cao S, Wang N, Zhou Z, Liu XM, Xu Y, Tian NX, Zhang Y, Wang J, Wang LP, Wang Y. Deactivation of excitatory neurons in the prelimbic cortex via Cdk5 promotes pain sensation and anxiety. Nat Commun. 2015; 6:7660. PMID: 26179626.
Article26. Zhang Z, Gadotti VM, Chen L, Souza IA, Stemkowski PL, Zamponi GW. Role of prelimbic GABAergic circuits in sensory and emotional aspects of neuropathic pain. Cell Rep. 2015; 12:752–759. PMID: 26212331.
Article27. Barthas F, Sellmeijer J, Hugel S, Waltisperger E, Barrot M, Yalcin I. The anterior cingulate cortex is a critical hub for pain-induced depression. Biol Psychiatry. 2015; 77:236–245. PMID: 25433903.
Article28. Gu L, Uhelski ML, Anand S, Romero-Ortega M, Kim YT, Fuchs PN, Mohanty SK. Pain inhibition by optogenetic activation of specific anterior cingulate cortical neurons. PLoS One. 2015; 10:e0117746. PMID: 25714399.
Article29. Kang SJ, Kwak C, Lee J, Sim SE, Shim J, Choi T, Collingridge GL, Zhuo M, Kaang BK. Bidirectional modulation of hyperalgesia via the specific control of excitatory and inhibitory neuronal activity in the ACC. Mol Brain. 2015; 8:81. PMID: 26631249.
Article30. Craig AD. A new view of pain as a homeostatic emotion. Trends Neurosci. 2003; 26:303–307. PMID: 12798599.31. Navratilova E, Porreca F. Reward and motivation in pain and pain relief. Nat Neurosci. 2014; 17:1304–1312. PMID: 25254980.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- αα-Pinene Attenuates Methamphetamine-Induced Conditioned Place Preference in C57BL/6 Mice
- A Critical Involvement of Glutamatergic Neurons in the Anterior Insular Cortex for Subdiaphragmatic Vagotomy-induced Analgesia
- Analgesic effects of soluble epoxide hydrolase inhibitor in K/BxN serum transfer arthritis mouse model
- Different development patterns of reward behaviors induced by ketamine and JWH-018 in striatal GAD67 knockdown mice
- T2 Relaxation Times of the Cingulate Cortex, Amygdaloid Body, Hippocampal Body, and Insular Cortex: Comparison of 1.5 T and 3.0 T