Immune Netw.
2017 Aug;17(4):201-213. 10.4110/in.2017.17.4.201.
T Cell's Sense of Self: a Role of Self-Recognition in Shaping Functional Competence of Naïve T Cells
- Affiliations
-
- 1Academy of Immunology and Microbiology, Institute for Basic Science, Pohang 37673, Korea. jhcho90@ibs.re.kr
- 2Division of Integrative Biosciences and Biotechnology, Pohang University of Science and Technology, Pohang 37673, Korea.
- KMID: 2388075
- DOI: http://doi.org/10.4110/in.2017.17.4.201
Abstract
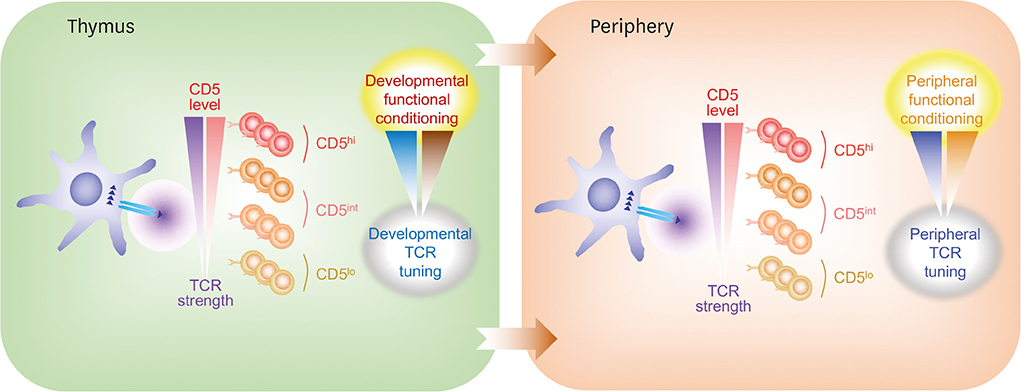
- Post-thymic naïve T cells constitute a key cellular arm of adaptive immunity, with a well-known characteristic of the specificity and robustness of responses to cognate foreign antigens which is presented as a form of antigen-derived peptides bound to major histocompatibility complex (MHC) molecules by antigen-presenting cells (APCs). In a steady state, however, these cells are resting, quiescent in their activity, but must keep full ranges of functional integrity to mount rapid and robust immunity to cope with various infectious pathogens at any time and space. Such unique property of resting naïve T cells is not acquired in a default manner but rather requires an active mechanism. Although our understanding of exactly how this process occurs and what factors are involved remains incomplete, a particular role of self-recognition by T cells has grown greatly in recent years. In this brief review, we discuss recent data on how the interaction of T cells with self-peptide MHC ligands regulates their functional responsiveness and propose that variable strength of self-reactivity imposes distinctly different levels of functional competence and heterogeneity.
Keyword
MeSH Terms
Figure
Reference
-
1. Jameson SC, Hogquist KA, Bevan MJ. Specificity and flexibility in thymic selection. Nature. 1994; 369:750–752.
Article2. Kisielow P, Miazek A. Thymic selection and tolerance. Transplant Proc. 1996; 28:3429–3430.3. Kruisbeek AM, Zúñiga-Pflücker J, Marusić-Galesić S, Weston MA, Tentori L, Longo DL. Thymic selection of the T-cell repertoire. Immunol Res. 1988; 7:318–328.
Article4. Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003; 21:139–176.
Article5. Klein L, Hinterberger M, Wirnsberger G, Kyewski B. Antigen presentation in the thymus for positive selection and central tolerance induction. Nat Rev Immunol. 2009; 9:833–844.
Article6. Palmer E, Naeher D. Affinity threshold for thymic selection through a T-cell receptor-co-receptor zipper. Nat Rev Immunol. 2009; 9:207–213.
Article7. Hogquist KA. Signal strength in thymic selection and lineage commitment. Curr Opin Immunol. 2001; 13:225–231.
Article8. Ladi E, Yin X, Chtanova T, Robey EA. Thymic microenvironments for T cell differentiation and selection. Nat Immunol. 2006; 7:338–343.
Article9. Gascoigne NR, Palmer E. Signaling in thymic selection. Curr Opin Immunol. 2011; 23:207–212.
Article10. Jameson SC. Maintaining the norm: T-cell homeostasis. Nat Rev Immunol. 2002; 2:547–556.
Article11. Surh CD, Sprent J. Homeostasis of naive and memory T cells. Immunity. 2008; 29:848–862.
Article12. Sprent J, Cho JH, Boyman O, Surh CD. T cell homeostasis. Immunol Cell Biol. 2008; 86:312–319.
Article13. Stefanová I, Dorfman JR, Germain RN. Self-recognition promotes the foreign antigen sensitivity of naive T lymphocytes. Nature. 2002; 420:429–434.
Article14. Mandl JN, Monteiro JP, Vrisekoop N, Germain RN. T cell-positive selection uses self-ligand binding strength to optimize repertoire recognition of foreign antigens. Immunity. 2013; 38:263–274.
Article15. Fulton RB, Hamilton SE, Xing Y, Best JA, Goldrath AW, Hogquist KA, Jameson SC. The TCR's sensitivity to self peptide-MHC dictates the ability of naive CD8(+) T cells to respond to foreign antigens. Nat Immunol. 2015; 16:107–117.
Article16. Fischer UB, Jacovetty EL, Medeiros RB, Goudy BD, Zell T, Swanson JB, Lorenz E, Shimizu Y, Miller MJ, Khoruts A, et al. MHC class II deprivation impairs CD4 T cell motility and responsiveness to antigen-bearing dendritic cells in vivo. Proc Natl Acad Sci USA. 2007; 104:7181–7186.
Article17. Hochweller K, Wabnitz GH, Samstag Y, Suffner J, Hämmerling GJ, Garbi N. Dendritic cells control T cell tonic signaling required for responsiveness to foreign antigen. Proc Natl Acad Sci USA. 2010; 107:5931–5936.
Article18. Revy P, Sospedra M, Barbour B, Trautmann A. Functional antigen-independent synapses formed between T cells and dendritic cells. Nat Immunol. 2001; 2:925–931.
Article19. Yachi PP, Ampudia J, Gascoigne NR, Zal T. Nonstimulatory peptides contribute to antigen-induced CD8-T cell receptor interaction at the immunological synapse. Nat Immunol. 2005; 6:785–792.
Article20. Krogsgaard M, Li QJ, Sumen C, Huppa JB, Huse M, Davis MM. Agonist/endogenous peptide-MHC heterodimers drive T cell activation and sensitivity. Nature. 2005; 434:238–243.
Article21. Yachi PP, Lotz C, Ampudia J, Gascoigne NR. T cell activation enhancement by endogenous pMHC acts for both weak and strong agonists but varies with differentiation state. J Exp Med. 2007; 204:2747–2757.
Article22. Ebert PJ, Jiang S, Xie J, Li QJ, Davis MM. An endogenous positively selecting peptide enhances mature T cell responses and becomes an autoantigen in the absence of microRNA miR-181a. Nat Immunol. 2009; 10:1162–1169.
Article23. Lo WL, Felix NJ, Walters JJ, Rohrs H, Gross ML, Allen PM. An endogenous peptide positively selects and augments the activation and survival of peripheral CD4+ T cells. Nat Immunol. 2009; 10:1155–1161.
Article24. Irvine DJ, Purbhoo MA, Krogsgaard M, Davis MM. Direct observation of ligand recognition by T cells. Nature. 2002; 419:845–849.
Article25. Tarakhovsky A, Kanner SB, Hombach J, Ledbetter JA, Müller W, Killeen N, Rajewsky K. A role for CD5 in TCR-mediated signal transduction and thymocyte selection. Science. 1995; 269:535–537.
Article26. Azzam HS, Grinberg A, Lui K, Shen H, Shores EW, Love PE. CD5 expression is developmentally regulated by T cell receptor (TCR) signals and TCR avidity. J Exp Med. 1998; 188:2301–2311.
Article27. Azzam HS, DeJarnette JB, Huang K, Emmons R, Park CS, Sommers CL, El-Khoury D, Shores EW, Love PE. Fine tuning of TCR signaling by CD5. J Immunol. 2001; 166:5464–5472.
Article28. Cho JH, Kim HO, Surh CD, Sprent J. T cell receptor-dependent regulation of lipid rafts controls naive CD8+ T cell homeostasis. Immunity. 2010; 32:214–226.
Article29. Persaud SP, Parker CR, Lo WL, Weber KS, Allen PM. Intrinsic CD4+ T cell sensitivity and response to a pathogen are set and sustained by avidity for thymic and peripheral complexes of self peptide and MHC. Nat Immunol. 2014; 15:266–274.
Article30. Wong P, Barton GM, Forbush KA, Rudensky AY. Dynamic tuning of T cell reactivity by self-peptide-major histocompatibility complex ligands. J Exp Med. 2001; 193:1179–1187.
Article31. Li QJ, Chau J, Ebert PJ, Sylvester G, Min H, Liu G, Braich R, Manoharan M, Soutschek J, Skare P, et al. miR-181a is an intrinsic modulator of T cell sensitivity and selection. Cell. 2007; 129:147–161.
Article32. Stephen TL, Wilson BS, Laufer TM. Subcellular distribution of Lck during CD4 T-cell maturation in the thymic medulla regulates the T-cell activation threshold. Proc Natl Acad Sci USA. 2012; 109:7415–7420.
Article33. Wiede F, La Gruta NL, Tiganis T. PTPN2 attenuates T-cell lymphopenia-induced proliferation. Nat Commun. 2014; 5:3073.
Article34. Salmond RJ, Brownlie RJ, Morrison VL, Zamoyska R. The tyrosine phosphatase PTPN22 discriminates weak self peptides from strong agonist TCR signals. Nat Immunol. 2014; 15:875–883.
Article35. Grossman Z, Paul WE. Dynamic tuning of lymphocytes: physiological basis, mechanisms, and function. Annu Rev Immunol. 2015; 33:677–713.
Article36. Peña-Rossi C, Zuckerman LA, Strong J, Kwan J, Ferris W, Chan S, Tarakhovsky A, Beyers AD, Killeen N. Negative regulation of CD4 lineage development and responses by CD5. J Immunol. 1999; 163:6494–6501.37. Chan S, Waltzinger C, Tarakhovsky A, Benoist C, Mathis D. An influence of CD5 on the selection of CD4-lineage T cells. Eur J Immunol. 1999; 29:2916–2922.38. Orta-Mascaró M, Consuegra-Fernández M, Carreras E, Roncagalli R, Carreras-Sureda A, Alvarez P, Girard L, Simões I, Martínez-Florensa M, Aranda F, et al. CD6 modulates thymocyte selection and peripheral T cell homeostasis. J Exp Med. 2016; 213:1387–1397.
Article39. Gimferrer I, Farnós M, Calvo M, Mittelbrunn M, Enrich C, Sánchez-Madrid F, Vives J, Lozano F. The accessory molecules CD5 and CD6 associate on the membrane of lymphoid T cells. J Biol Chem. 2003; 278:8564–8571.
Article40. Smith K, Seddon B, Purbhoo MA, Zamoyska R, Fisher AG, Merkenschlager M. Sensory adaptation in naive peripheral CD4 T cells. J Exp Med. 2001; 194:1253–1261.
Article41. Bhandoola A, Tai X, Eckhaus M, Auchincloss H, Mason K, Rubin SA, Carbone KM, Grossman Z, Rosenberg AS, Singer A. Peripheral expression of self-MHC-II influences the reactivity and self-tolerance of mature CD4(+) T cells: evidence from a lymphopenic T cell model. Immunity. 2002; 17:425–436.
Article42. Takada K, Jameson SC. Self-class I MHC molecules support survival of naive CD8 T cells, but depress their functional sensitivity through regulation of CD8 expression levels. J Exp Med. 2009; 206:2253–2269.
Article43. Cho JH, Kim HO, Ju YJ, Kye YC, Lee GW, Lee SW, Yun CH, Bottini N, Webster K, Goodnow CC, et al. CD45-mediated control of TCR tuning in naïve and memory CD8(+) T cells. Nat Commun. 2016; 7:13373.
Article44. Teh SJ, Killeen N, Tarakhovsky A, Littman DR, Teh HS. CD2 regulates the positive selection and function of antigen-specific CD4- CD8+ T cells. Blood. 1997; 89:1308–1318.
Article45. Hawiger D, Inaba K, Dorsett Y, Guo M, Mahnke K, Rivera M, Ravetch JV, Steinman RM, Nussenzweig MC. Dendritic cells induce peripheral T cell unresponsiveness under steady state conditions in vivo. J Exp Med. 2001; 194:769–779.46. Bonifaz L, Bonnyay D, Mahnke K, Rivera M, Nussenzweig MC, Steinman RM. Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J Exp Med. 2002; 196:1627–1638.
Article47. Probst HC, Lagnel J, Kollias G, van den Broek M. Inducible transgenic mice reveal resting dendritic cells as potent inducers of CD8+ T cell tolerance. Immunity. 2003; 18:713–720.
Article48. Probst HC, McCoy K, Okazaki T, Honjo T, van den Broek M. Resting dendritic cells induce peripheral CD8+ T cell tolerance through PD-1 and CTLA-4. Nat Immunol. 2005; 6:280–286.
Article49. Luckashenak N, Schroeder S, Endt K, Schmidt D, Mahnke K, Bachmann MF, Marconi P, Deeg CA, Brocker T. Constitutive crosspresentation of tissue antigens by dendritic cells controls CD8+ T cell tolerance in vivo. Immunity. 2008; 28:521–532.
Article50. Lee JW, Epardaud M, Sun J, Becker JE, Cheng AC, Yonekura AR, Heath JK, Turley SJ. Peripheral antigen display by lymph node stroma promotes T cell tolerance to intestinal self. Nat Immunol. 2007; 8:181–190.
Article51. Fletcher AL, Lukacs-Kornek V, Reynoso ED, Pinner SE, Bellemare-Pelletier A, Curry MS, Collier AR, Boyd RL, Turley SJ. Lymph node fibroblastic reticular cells directly present peripheral tissue antigen under steady-state and inflammatory conditions. J Exp Med. 2010; 207:689–697.
Article52. Cohen JN, Guidi CJ, Tewalt EF, Qiao H, Rouhani SJ, Ruddell A, Farr AG, Tung KS, Engelhard VH. Lymph node-resident lymphatic endothelial cells mediate peripheral tolerance via Aire-independent direct antigen presentation. J Exp Med. 2010; 207:681–688.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Chemotherapy With Androgen Deprivation for Hormone-Naïve Prostate Cancer
- Effects of the Core Competency Support Program in Parenting Role on Sense of Competence in Parenting, Parent-Child Communication, and Parental Role Satisfaction in Parents of Adolescents
- Influence of Spousal Support on the Relationship between Acculturative Stress and Sense of Parenting Competence among Married Vietnamese Immigrant Women
- Impact of Rumination, and Dyadic Coping on Parenting Sense of Competence Among Puerperal Women in China: A Cross-sectional Study
- The Impact of Impulsivity on Quality of Life in Early Drug-Naïve Parkinson's Disease Patients