J Korean Neurosurg Soc.
2017 Jul;60(4):391-396. 10.3340/jkns.2016.1010.018.
Ginkgolide B Modulates BDNF Expression in Acute Ischemic Stroke
- Affiliations
-
- 1Department of Neurology, Affiliated Provincial Hospital of Anhui Medical University, Hefei, China.
- 2Department of Neurology, Nanjing General Hospital of Nanjing Command, Nanjing, China.
- 3Department of Neurology, the First Hospital of Anhui Medical University, Hefei, China. wangkai230001@sina.com
- KMID: 2387876
- DOI: http://doi.org/10.3340/jkns.2016.1010.018
Abstract
OBJECTIVE
To investigate the neuroprotective effects of Ginkgolide B (GB) against ischemic stroke-induced injury in vivo and in vitro, and further explore the possible mechanisms concerned.
METHODS
Transient middle cerebral artery occlusion (tMCAO) mice and oxygen-glucose deprivation/reoxygenation (OGD/R)-treated N2a cells were used to explore the neuroprotective effects of GB. The expression of brain-derived neurotrophic factor (BDNF) was detected via Western blot and qRT-PCR.
RESULTS
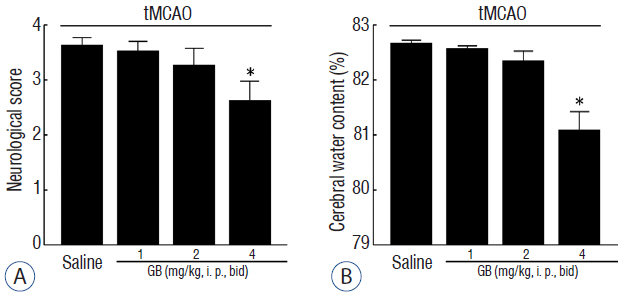
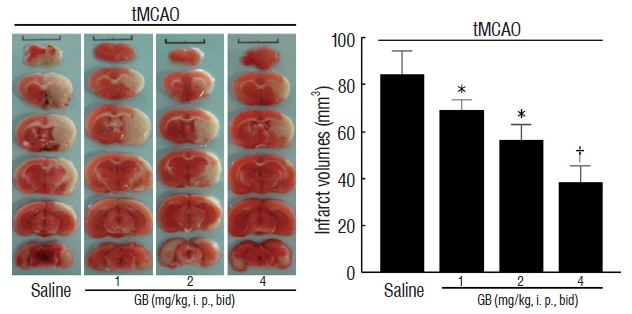
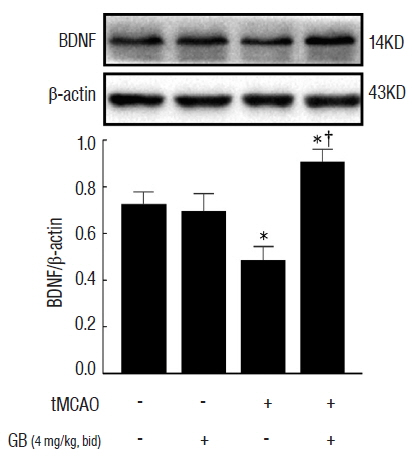
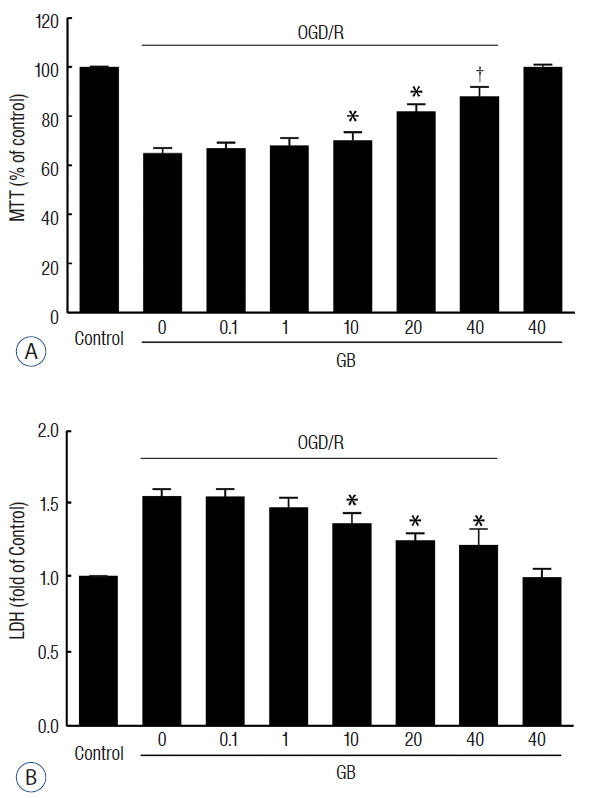
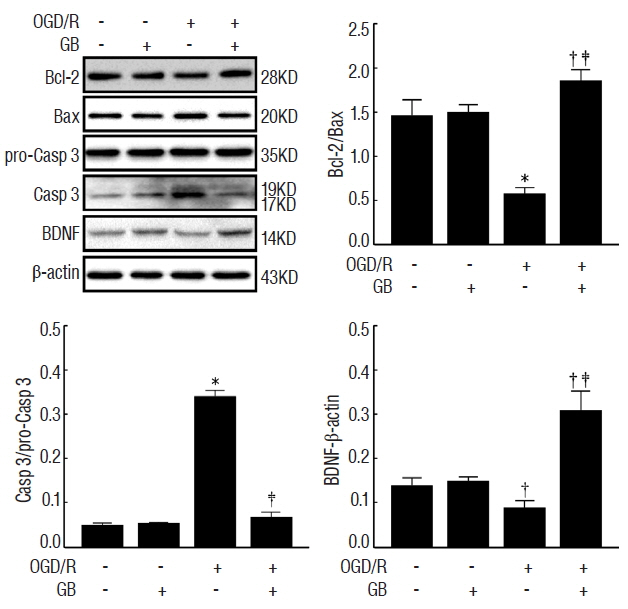
GB treatment (4 mg/kg, i. p., bid) significantly reduced neurological deficits, water content, and cerebral infarct volume in tMCAO mice. GB also significantly increased Bcl-2/Bax ratio, reduced the expression of caspase-3, and protected against OGD/R-induced neuronal apoptosis. Meanwhile, GB caused the up-regulation of BDNF protein in vivo and in vitro.
CONCLUSION
Our data suggest that GB might protect the brain against ischemic insult partly via modulating BDNF expression.
MeSH Terms
Figure
Reference
-
References
1. Albers GW, Goldstein LB, Hess DC, Wechsler LR, Furie KL, Gorelick PB, et al. Stroke Treatment Academic Industry Roundtable (STAIR) recommendations for maximizing the use of intravenous thrombolytics and expanding treatment options with intra-arterial and neuroprotective therapies. Stroke. 42:2645–2650. 2011.
Article2. Bederson JB, Pitts LH, Tsuji M, Nishimura MC, Davis RL, Bartkowski H. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 17:472–476. 1986.
Article3. Bekinschtein P, Cammarota M, Medina JH. BDNF and memory processing. Neuropharmacology. 76 Pt C:677–683. 2014.
Article4. Berretta A, Tzeng YC, Clarkson AN. Post-stroke recovery: the role of activity-dependent release of brain-derived neurotrophic factor. Expert Rev Neurother. 14:1335–1344. 2014.
Article5. Braquet P. Proofs of involvement of PAF-acether in various immune disorders using BN 52021 (ginkgolide B): a powerful PAF-acether antagonist isolated from Ginkgo biloba L. Adv Prostaglandin Thromboxane Leukot Res. 16:179–198. 1986.6. Chen PH, Gao S, Wang YJ, Xu AD, Li YS, Wang D. Classifying ischemic stroke, from TOAST to CISS. CNS Neurosci Ther. 18:452–456. 2012.
Article7. Desquand S, Touvay C, Randon J, Lagente V, Vilain B, Maridonneau-Parini I, et al. Interference of BN 52021 (ginkgolide B) with the broncho-pulmonary effects of PAF-acether in the guinea-pig. Eur J Pharmacol. 127:83–95. 1986.
Article8. Hatashita S, Hoff JT, Salamat SM. Ischemic brain edema and the osmotic gradient between blood and brain. J Cereb Blood Flow Metab. 8:552–559. 1988.
Article9. Hirouchi M, Itoh Y, Aoki Y, Oka M, Ukai Y. Reversal by NS-7, a neuroprotective compound, of the decrease in transcription factor CREB mRNA expression in rat brain after permanent middle cerebral artery occlusion. Neurosci Lett. 305:193–196. 2001.
Article10. Hou Y, Aboukhatwa MA, Lei DL, Manaye K, Khan I, Luo Y. Anti-depressant natural flavonols modulate BDNF and beta amyloid in neurons and hippocampus of double TgAD mice. Neuropharmacology. 58:911–920. 2010.
Article11. Ke Z, Yip SP, Li L, Zheng XX, Tong KY. The effects of voluntary, involuntary, and forced exercises on brain-derived neurotrophic factor and motor function recovery: a rat brain ischemia model. PLoS One. 6:e16643. 2011.
Article12. Larsson E, Nanobashvili A, Kokaia Z, Lindvall O. Evidence for neuroprotective effects of endogenous brain-derived neurotrophic factor after global forebrain ischemia in rats. J Cereb Blood Flow Metab. 19:1220–1228. 1999.
Article13. Panja D, Bramham CR. BDNF mechanisms in late LTP formation: a synthesis and breakdown. Neuropharmacology. 76 Pt C:664–676. 2014.
Article14. Qi D, Ouyang C, Wang Y, Zhang S, Ma X, Song Y, et al. HO-1 attenuates hippocampal neurons injury via the activation of BDNF-TrkB-PI3K/Akt signaling pathway in stroke. Brain Res. 1577:69–76. 2014.
Article15. Qin XS, Jin KH, Ding BK, Xie SF, Ma H. Effects of extract of Ginkgo biloba with venlafaxine on brain injury in a rat model of depression. Chin Med J (Engl). 118:391–397. 2005.16. Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 361:1545–1564. 2006.
Article17. Schäbitz WR, Sommer C, Zoder W, Kiessling M, Schwaninger M, Schwab S. Intravenous brain-derived neurotrophic factor reduces infarct size and counterregulates Bax and Bcl-2 expression after temporary focal cerebral ischemia. Stroke. 31:2212–2217. 2000.
Article18. Schäbitz WR, Steigleder T, Cooper-Kuhn CM, Schwab S, Sommer C, Schneider A, et al. Intravenous brain-derived neurotrophic factor enhances poststroke sensorimotor recovery and stimulates neurogenesis. Stroke. 38:2165–2172. 2007.
Article19. Seth P, Kumari R, Dikshit M, Srimal RC. Effect of platelet activating factor antagonists in different models of thrombosis. Thromb Res. 76:503–512. 1994.
Article20. Smith PF, Maclennan K, Darlington CL. The neuroprotective properties of the Ginkgo biloba leaf: a review of the possible relationship to platelet-activating factor (PAF). J Ethnopharmacol. 50:131–139. 1996.
Article21. Teather LA, Afonso VM, Wurtman RJ. Inhibition of platelet-activating factor receptors in hippocampal plasma membranes attenuates the inflammatory nociceptive response in rats. Brain Res. 1097:230–233. 2006.
Article22. Vargaftig BB, Lefort J, Chignard M, Benveniste J. Platelet-activating factor induces a platelet-dependent bronchoconstriction unrelated to the formation of prostaglandin derivatives. Eur J Pharmacol. 65:185–192. 1980.
Article23. Yin B, Xu Y, Wei R, Luo B. Ginkgo biloba on focal cerebral ischemia: a systematic review and meta-analysis. Am J Chin Med. 42:769–783. 2014.
Article24. Zhou J, Ping FF, Lv WT, Feng JY, Shang J. Interleukin-18 directly protects cortical neurons by activating PI3K/AKT/NF-κB/CREB pathways. Cytokine. 69:29–38. 2014.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Antiplatelet Therapy for Secondary Stroke Prevention in Patients with Ischemic Stroke or Transient Ischemic Attack
- Pneumococcal meningitis complicated by otomastoiditis and pneumocephalus confounding an acute ischemic stroke diagnosis
- Early In-hospital Management of Acute Ischemic Stroke
- PXR Mediated Protection against Liver Inflammation by Ginkgolide A in Tetrachloromethane Treated Mice
- Multiple Territory Ischemic Stroke Aggravated by Severe Anemia