Allergy Asthma Respir Dis.
2017 Jul;5(4):193-199. 10.4168/aard.2017.5.4.193.
The association between Staphylococcus aureus colonization and food sensitization in children with atopic dermatitis
- Affiliations
-
- 1Department of Pediatrics, Busan St. Mary's Hospital, Busan, Korea. hyh190@naver.com
- 2Department of Laboratory Medicine, Busan St. Mary's Hospital, Busan, Korea.
- KMID: 2387646
- DOI: http://doi.org/10.4168/aard.2017.5.4.193
Abstract
- PURPOSE
Atopic dermatitis is often accompanied by food allergies which occur through skin barrier defects. Especially Staphylococcus aureus colonization can exacerbate skin barrier defects that cause sensitization and increase specific IgE (sIgE) to food. We investigated the association between skin colonization and food sIgE changes in children with atopic dermatitis.
METHODS
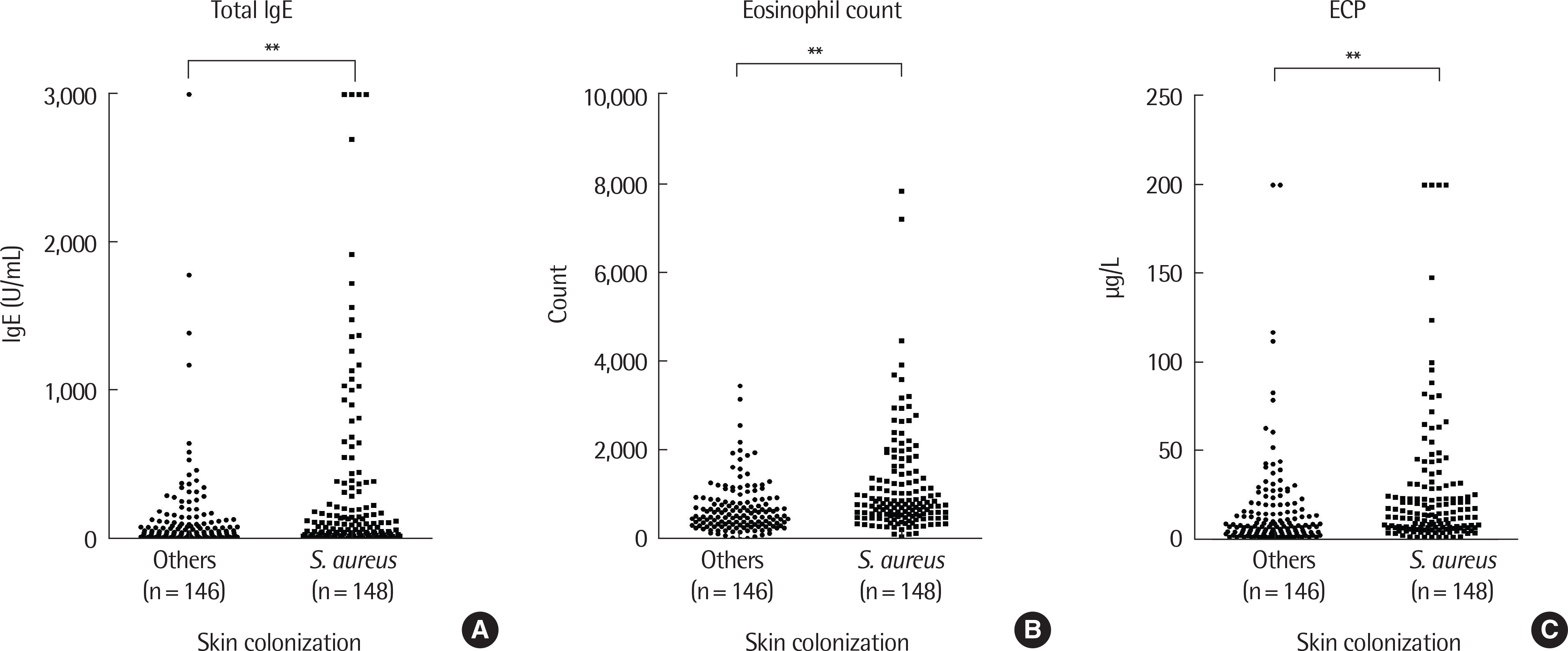
Atopic dermatitis was diagnosed by a pediatric allergist in patients between 3 months and 3 years of age. Total IgE and sIgE to egg white, cow's milk, wheat, and peanuts were taken. Eosinophil count and eosinophil cationic protein were also taken. Comparisons were done between the groups with and without S. aureus colonization.
RESULTS
It was found that 50.3% of the 294 enrolled patients had S. aureus colonization on lesional skin. Statistically significant sensitization to wheat and peanut were increased with S. aureus colonization. Statistically significant increases in sIgE (above cutoff level) were also found in egg white, milk, wheat and peanut. Higher S. aureus colony counts also increased sIgE of all foods. Methicillin-resistant S. aureus showed no statistical difference compared to methicillin-susceptible S. aureus in severity and sIgE levels.
CONCLUSION
S. aureus colonization increases the risk of food sensitization in children with atopic dermatitis.
MeSH Terms
Figure
Reference
-
1. Flohr C, Mann J. New insights into the epidemiology of childhood atopic dermatitis. Allergy. 2014; 69:3–16.
Article2. Boyce JA, Assa'ad A, Burks AW, Jones SM, Sampson HA, Wood RA, et al. Guidelines for the diagnosis and management of food allergy in the United States: summary of the NIAID-Sponsored Expert Panel Report. J Allergy Clin Immunol. 2010; 126:1105–18.
Article3. Werfel T, Breuer K. Role of food allergy in atopic dermatitis. Curr Opin Allergy Clin Immunol. 2004; 4:379–85.
Article4. Leung DY, Guttman-Yassky E. Deciphering the complexities of atopic dermatitis: shifting paradigms in treatment approaches. J Allergy Clin Immunol. 2014; 134:769–79.
Article5. Foster TJ. Immune evasion by staphylococci. Nat Rev Microbiol. 2005; 3:948–58.
Article6. McFadden JP, Noble WC, Camp RD. Superantigenic exotoxin-secreting potential of staphylococci isolated from atopic eczematous skin. Br J Dermatol. 1993; 128:631–2.
Article7. Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol (Stockh). 1980; 92:44–7.8. Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001; 107:891–6.
Article9. Goodyear HM, Watson PJ, Egan SA, Price EH, Kenny PA, Harper JI. Skin microflora of atopic eczema in first time hospital attenders. Clin Exp Dermatol. 1993; 18:300–4.
Article10. Lipnharski C, d'Azevedo PA, Quinto VP, Bessa G, Bonamigo RR. Colonization by S. aureus increases the EASI and the number of appointments by patients with atopic dermatitis: cohort with 93 patients. An Bras Dermatol. 2013; 88:518–21.11. Jagadeesan S, Kurien G, Divakaran MV, Sadanandan SM, Sobhanaku-mari K, Sarin A. Methicillin-resistant Staphylococcus aureus colonization and disease severity in atopic dermatitis: a cross-sectional study from South India. Indian J Dermatol Venereol Leprol. 2014; 80:229–34.
Article12. Hon KL, Tsang YC, Pong NH, Ng C, Ip M, Leung TF. Clinical features and Staphylococcus aureus colonization/infection in childhood atopic dermatitis. J Dermatolog Treat. 2016; 27:235–40.13. Kobayashi T, Glatz M, Horiuchi K, Kawasaki H, Akiyama H, Kaplan DH, et al. Dysbiosis and Staphylococcus aureus colonization drives inflammation in atopic dermatitis. Immunity. 2015; 42:756–66.
Article14. Jones AL, Curran-Everett D, Leung DY. Food allergy is associated with Staphylococcus aureus colonization in children with atopic dermatitis. J Allergy Clin Immunol. 2016; 137:1247–8.e1-3.
Article15. Lack G. Update on risk factors for food allergy. J Allergy Clin Immunol. 2012; 129:1187–97.
Article16. Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002; 347:1151–60.
Article17. Schlievert PM, Strandberg KL, Lin YC, Peterson ML, Leung DY. Secreted virulence factor comparison between methicillin-resistant and methicil-lin-sensitive Staphylococcus aureus, and its relevance to atopic dermatitis. J Allergy Clin Immunol. 2010; 125:39–49.
Article18. Strange P, Skov L, Lisby S, Nielsen PL, Baadsgaard O. Staphylococcal enterotoxin B applied on intact normal and intact atopic skin induces dermatitis. Arch Dermatol. 1996; 132:27–33.
Article19. Ganeshan K, Neilsen CV, Hadsaitong A, Schleimer RP, Luo X, Bryce PJ. Impairing oral tolerance promotes allergy and anaphylaxis: a new murine food allergy model. J Allergy Clin Immunol. 2009; 123:231–8.e4.
Article20. Neuber K, Steinrücke K, Ring J. Staphylococcal enterotoxin B affects in vitro IgE synthesis, interferon-gamma, interleukin-4 and interleukin-5 production in atopic eczema. Int Arch Allergy Immunol. 1995; 107:179–82.21. Forbes EE, Camberis M, Prout M, Tang S, Paul WE, Le Gros G. Bacterial superantigen elicits food-induced allergic sensitization through epicuta-neous exposure. J Allergy Clin Immunol. 2010; 125(2 Suppl 1):AB113.
Article22. Hauk PJ, Hamid QA, Chrousos GP, Leung DY. Induction of corticosteroid insensitivity in human PBMCs by microbial superantigens. J Allergy Clin Immunol. 2000; 105:782–7.
Article23. Komata T, Söderström L, Borres MP, Tachimoto H, Ebisawa M. The predictive relationship of food-specific serum IgE concentrations to challenge outcomes for egg and milk varies by patient age. J Allergy Clin Immunol. 2007; 119:1272–4.
Article24. Ando H, Movérare R, Kondo Y, Tsuge I, Tanaka A, Borres MP, et al. Utility of ovomucoid-specific IgE concentrations in predicting symptomatic egg allergy. J Allergy Clin Immunol. 2008; 122:583–8.
Article25. Bath-Hextall FJ, Birnie AJ, Ravenscroft JC, Williams HC. Interventions to reduce Staphylococcus aureus in the management of atopic eczema: an updated Cochrane review. Br J Dermatol. 2010; 163:12–26.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Interactions Between Atopic Dermatitis and Staphylococcus aureus Infection: Clinical Implications
- Colonization of Staphylococcus aureus and sensitivity to antibiotics in children with atopic dermatitis
- The Effect of Antigen Sensitization and Development of Respiratory Allergy Disease on Severity of Atopic Dermatitis
- Relationship between Allergen Sensitization and Frequency of Asthma in Preschool Atopic Dermatitis Children
- The role of antiseptic agents in atopic dermatitis